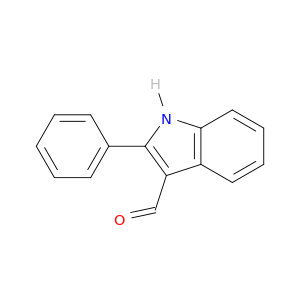
2-Phenylindole-3-carboxaldehyde
| Title | Journal |
|---|---|
| Synthesis of 2-arylindole derivatives and evaluation as nitric oxide synthase and NFκB inhibitors. | Organic & biomolecular chemistry 20121128 |
| Structural findings of 2-phenylindole-3-carbaldehyde derivatives for antimitotic activity by FA-sMLR QSAR analysis. | Chemical biology & drug design 20100201 |
| CoMFA and docking studies of 2-phenylindole derivatives with anticancer activity. | European journal of medicinal chemistry 20090701 |
| Comparative QSAR modelling of 2-phenylindole-3-carbaldehyde derivatives as potential antimitotic agents. | Bioorganic & medicinal chemistry letters 20090315 |
| [(2-Phenylindol-3-yl)methylene]propanedinitriles inhibit the growth of breast cancer cells by cell cycle arrest in G(2)/M phase and apoptosis. | Bioorganic & medicinal chemistry 20071201 |