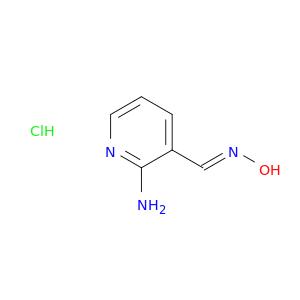
2-Amino-pyridine-3-carbaldehyde oxime, HCl
| Title | Journal |
|---|---|
| Detection of enzymatically generated hydrogen peroxide by metal-based fluorescent probe. | Analytical chemistry 20111215 |
| Laser-induced mixing in microfluidic channels. | Analytical chemistry 20070615 |
| Measurement of enzyme kinetics using a continuous-flow microfluidic system. | Analytical chemistry 20030701 |
| A fluorometric assay for detection of lysyl oxidase enzyme activity in biological samples. | Analytical biochemistry 20020115 |
| In vitro quantitation of biological superoxide and hydrogen peroxide generation. | Methods in enzymology 20020101 |
| Hydrogen peroxide formation during iron deposition in horse spleen ferritin using O2 as an oxidant. | Biochemistry 20010320 |