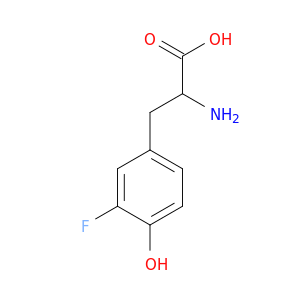
2-amino-3-(3-fluoro-4-hydroxyphenyl)propanoic acid
| Title | Journal |
|---|---|
| 3-Fluorotyrosine as a complementary probe of hemoglobin structure and dynamics: a (19)F-NMR study of Synechococcus sp. PCC 7002 GlbN. | Chemistry & biodiversity 20120901 |
| Site-specific incorporation of fluorotyrosines into proteins in Escherichia coli by photochemical disguise. | Biochemistry 20100302 |
| Approaches for the measurement of solvent exposure in proteins by 19F NMR. | Journal of biomolecular NMR 20091101 |
| 19F NMR studies of alpha-synuclein conformation and fibrillation. | Biochemistry 20090915 |
| Comparison of the folding mechanism of highly homologous proteins in the lipid-binding protein family. | Proteins 20090601 |
| Probing the coupling between proton and electron transfer in photosystem II core complexes containing a 3-fluorotyrosine. | Journal of the American Chemical Society 20090401 |
| A mutagenesis-free approach to assignment of (19)F NMR resonances in biosynthetically labeled proteins. | Journal of the American Chemical Society 20090218 |
| Site-specific incorporation of fluorotyrosines into the R2 subunit of E. coli ribonucleotide reductase by expressed protein ligation. | Nature protocols 20070101 |
| Electron transfer reactions of fluorotyrosyl radicals. | Journal of the American Chemical Society 20061025 |
| Aminoacrylate intermediates in the reaction of Citrobacter freundii tyrosine phenol-lyase. | Biochemistry 20060808 |
| Structural mobility in human manganese superoxide dismutase. | Biochemistry 20060711 |
| Mono-, di-, tri-, and tetra-substituted fluorotyrosines: new probes for enzymes that use tyrosyl radicals in catalysis. | Journal of the American Chemical Society 20060208 |
| Hydrogen bonding in human manganese superoxide dismutase containing 3-fluorotyrosine. | Biophysical journal 20051201 |
| Structural and spectral response of Aequorea victoria green fluorescent proteins to chromophore fluorination. | Biochemistry 20050315 |
| Crystallographic evidence for isomeric chromophores in 3-fluorotyrosyl-green fluorescent protein. | Chembiochem : a European journal of chemical biology 20040503 |
| Evaluating the potential of fluorinated tyrosines as spectroscopic probes of local protein environments: a UV resonance Raman study. | Biochemistry 20030304 |
| In vivo metabolism and partitioning of 6-[18F]fluoro-L-meta-tyrosine in whole blood: a unified compartment model. | Physics in medicine and biology 20020607 |