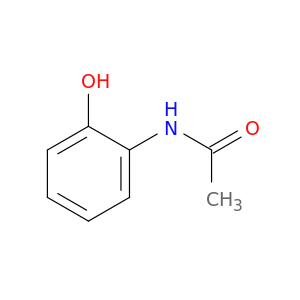
2-Acetamidophenol
| Title | Journal |
|---|---|
| Acetaminophen-induced differentiation of human breast cancer stem cells and inhibition of tumor xenograft growth in mice. | Biochemical pharmacology 20110501 |
| Synthesis and SAR studies of 1,4-benzoxazine MenB inhibitors: novel antibacterial agents against Mycobacterium tuberculosis. | Bioorganic & medicinal chemistry letters 20101101 |
| N-(2-hydroxy phenyl) acetamide inhibits inflammation-related cytokines and ROS in adjuvant-induced arthritic (AIA) rats. | International immunopharmacology 20100801 |
| Hydroxyl-functionalized polyaniline nanospheres: tracing molecular interactions at the nanosurface via vitamin C sensing. | Langmuir : the ACS journal of surfaces and colloids 20080902 |
| Interactions of Bacillus mojavensis and Fusarium verticillioides with a benzoxazolinone (BOA) and its transformation product, APO. | Journal of chemical ecology 20071001 |
| Zea mays: benzoxazolinone detoxification under sulfur deficiency conditions--a complex allelopathic alliance including endophytic Fusarium verticillioides. | Journal of chemical ecology 20070201 |
| Amorphane sesquiterpenes from a marine Streptomyces sp. | Journal of natural products 20070201 |
| Microsphere-based protease assays and screening application for lethal factor and factor Xa. | Cytometry. Part A : the journal of the International Society for Analytical Cytology 20060501 |
| Joint action of benzoxazinone derivatives and phenolic acids. | Journal of agricultural and food chemistry 20060222 |
| Method for screening and quantitative determination of serum levels of salicylic Acid, acetaminophen, theophylline, phenobarbital, bromvalerylurea, pentobarbital, and amobarbital using liquid chromatography/electrospray mass spectrometry. | Biological & pharmaceutical bulletin 20060101 |
| Application of stable isotope labeled glutathione and rapid scanning mass spectrometers in detecting and characterizing reactive metabolites. | Rapid communications in mass spectrometry : RCM 20050101 |
| Analysis of paraquat, diquat and two diquat metabolites in biological materials by high-performance liquid chromatography. | Legal medicine (Tokyo, Japan) 20020901 |
| Hydroxylated 2-amino-3H-phenoxazin-3-one derivatives as products of 2-hydroxy-1,4-benzoxazin-3-one (HBOA) biotransformation by Chaetosphaeria sp., an endophytic fungus from Aphelandra tetragona. | Zeitschrift fur Naturforschung. C, Journal of biosciences 20020101 |