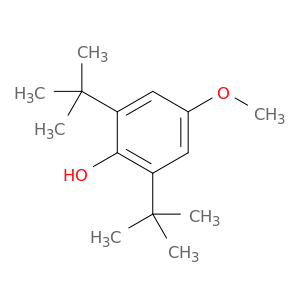
2,6-Di-tert-butyl-4-methoxyphenol
| Title | Journal |
|---|---|
| Discovery of novel SERCA inhibitors by virtual screening of a large compound library. | European journal of medicinal chemistry 20110501 |
| Concerted proton-electron transfer in a ruthenium terpyridyl-benzoate system with a large separation between the redox and basic sites. | Journal of the American Chemical Society 20090729 |
| Carbonic anhydrase inhibitors. Inhibition of human erythrocyte isozymes I and II with a series of antioxidant phenols. | Bioorganic & medicinal chemistry 20090415 |
| Concise synthesis of 1,2-dihydroisoquinolines and 1H-isochromenes by carbophilic lewis acid-catalyzed tandem nucleophilic addition and cyclization of 2-(1-alkynyl)arylaldimines and 2-(1-alkynyl)arylaldehydes. | The Journal of organic chemistry 20070608 |
| Radically different antioxidants: thermally generated carbon-centered radicals as chain-breaking antioxidants. | Journal of the American Chemical Society 20061227 |
| Polypyrroles as antioxidants: kinetic studies on reactions of bilirubin and biliverdin dimethyl esters and synthetic model compounds with peroxyl radicals in solution. Chemical calculations on selected typical structures. | The Journal of organic chemistry 20060106 |
| A quantitative approach to the free radical interaction between alpha-tocopherol and the coantioxidants eugenol, resveratrol or ascorbate. | In vivo (Athens, Greece) 20060101 |
| Yucca schidigera bark: phenolic constituents and antioxidant activity. | Journal of natural products 20040501 |
| Kinetic studies on stilbazulenyl-bis-nitrone (STAZN), a nonphenolic chain-breaking antioxidant in solution, micelles, and lipid membranes. | The Journal of organic chemistry 20040430 |
| 3,5-di-t-butyl-4-hydroxyanisole (DTBHA) activation of rat skeletal muscle sarcoplasmic reticulum Ca(2+)-ATPase. | Biochemical pharmacology 20011215 |
| Suillusin, a unique benzofuran from the mushroom Suillus granulatus. | Journal of natural products 20010901 |
| Effects of some sterically hindered phenols on whole-cell Ca(2+) current of guinea-pig gastric fundus smooth muscle cells. | British journal of pharmacology 20010301 |