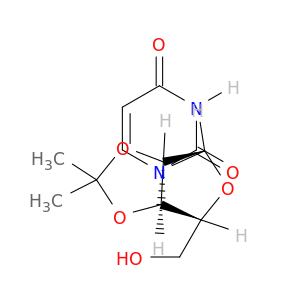
2',3'-O-Isopropylideneuridine
| Title | Journal |
|---|---|
| 5'-Uridyl derivatives of N-glycosyl allophanic acid and biuret. | Carbohydrate research 20100111 |
| Microsphere-based protease assays and screening application for lethal factor and factor Xa. | Cytometry. Part A : the journal of the International Society for Analytical Cytology 20060501 |
| Synthesis of N3-substituted uridine and related pyrimidine nucleosides and their antinociceptive effects in mice. | Chemical & pharmaceutical bulletin 20050301 |
| Sugar-modified conjugated diene analogues of adenosine and uridine: synthesis, interaction with S-adenosyl-L-homocysteine hydrolase, and antiviral and cytostatic effects. | Journal of medicinal chemistry 20020606 |