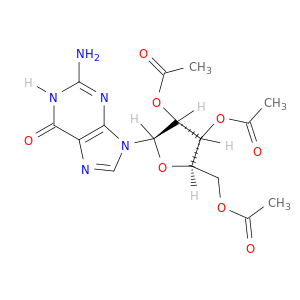
2',3',5'-Triacetylguanosine
| Title | Journal |
|---|---|
| Structure and gelation mechanism of tunable guanosine-based supramolecular hydrogels. | Langmuir : the ACS journal of surfaces and colloids 20100615 |
| Oxidative modification of guanine bases initiated by oxyl radicals derived from photolysis of azo compounds. | The journal of physical chemistry. B 20100520 |
| Tailoring the properties of guanosine-based supramolecular hydrogels. | Langmuir : the ACS journal of surfaces and colloids 20090804 |
| Pathways of arachidonic acid peroxyl radical reactions and product formation with guanine radicals. | Chemical research in toxicology 20080201 |
| Oxidative generation of guanine radicals by carbonate radicals and their reactions with nitrogen dioxide to form site specific 5-guanidino-4-nitroimidazole lesions in oligodeoxynucleotides. | Chemical research in toxicology 20030801 |
| Peroxynitrite-induced reactions of synthetic oligo 2'-deoxynucleotides and DNA containing guanine: formation and stability of a 5-guanidino-4-nitroimidazole lesion. | Biochemistry 20020611 |
| A novel nitroimidazole compound formed during the reaction of peroxynitrite with 2',3',5'-tri-O-acetyl-guanosine. | Journal of the American Chemical Society 20011212 |