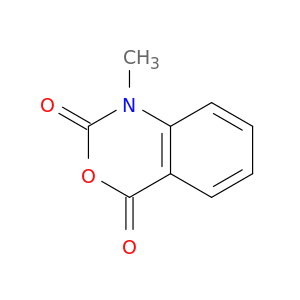
1-Methyl-1H-benzo[d][1,3]oxazine-2,4-dione
| Title | Journal |
|---|---|
| Identification and development of the 1,4-benzodiazepin-2-one and quinazoline-2,4-dione scaffolds as submicromolar inhibitors of HAT. | Bioorganic & medicinal chemistry 20121015 |
| Fingerprinting noncanonical and tertiary RNA structures by differential SHAPE reactivity. | Journal of the American Chemical Society 20120815 |
| Synthesis and characterization of a fluorescent analogue of cyclic di-GMP. | Biochemistry 20120710 |
| Automated RNA structure prediction uncovers a kink-turn linker in double glycine riboswitches. | Journal of the American Chemical Society 20120125 |
| Probing Retroviral and Retrotransposon Genome Structures: The 'SHAPE' of Things to Come. | Molecular biology international 20120101 |
| RNA folding and catalysis mediated by iron (II). | PloS one 20120101 |
| A universal pathway for kinesin stepping. | Nature structural & molecular biology 20110901 |
| Use of specific chemical reagents for detection of modified nucleotides in RNA. | Journal of nucleic acids 20110101 |
| 1-Methyl-4H-3,1-benzoxazine-2,4(1H)dione. | Acta crystallographica. Section E, Structure reports online 20100301 |
| An upstream Hfq binding site in the fhlA mRNA leader region facilitates the OxyS-fhlA interaction. | PloS one 20100101 |
| A lanthanide-based chemosensor for bioavailable Fe3+ using a fluorescent siderophore: an assay displacement approach. | Sensors (Basel, Switzerland) 20100101 |
| Revisiting plus-strand DNA synthesis in retroviruses and long terminal repeat retrotransposons: dynamics of enzyme: substrate interactions. | Viruses 20091201 |
| SHAMS: combining chemical modification of RNA with mass spectrometry to examine polypurine tract-containing RNA/DNA hybrids. | RNA (New York, N.Y.) 20090801 |
| Influence of nucleotide identity on ribose 2'-hydroxyl reactivity in RNA. | RNA (New York, N.Y.) 20090701 |
| Ligand-dependent folding of the three-way junction in the purine riboswitch. | RNA (New York, N.Y.) 20080401 |
| High-throughput SHAPE analysis reveals structures in HIV-1 genomic RNA strongly conserved across distinct biological states. | PLoS biology 20080401 |
| Simultaneous determination of myo-inositol and scyllo-inositol by MEKC as a rapid monitoring tool for inositol levels. | Electrophoresis 20070401 |
| Microsphere-based protease assays and screening application for lethal factor and factor Xa. | Cytometry. Part A : the journal of the International Society for Analytical Cytology 20060501 |
| Selective 2'-hydroxyl acylation analyzed by primer extension (SHAPE): quantitative RNA structure analysis at single nucleotide resolution. | Nature protocols 20060101 |
| RNA SHAPE chemistry reveals nonhierarchical interactions dominate equilibrium structural transitions in tRNA(Asp) transcripts. | Journal of the American Chemical Society 20050406 |
| RNA structure analysis at single nucleotide resolution by selective 2'-hydroxyl acylation and primer extension (SHAPE). | Journal of the American Chemical Society 20050330 |
| Are isatin and isatoic anhydride antiaromatic and aromatic respectively? A combined experimental and theoretical investigation. | Organic & biomolecular chemistry 20030721 |