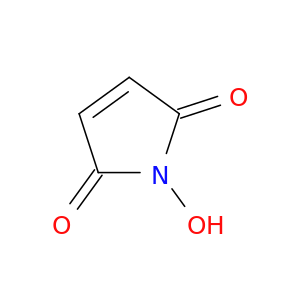
1-Hydroxy-1H-pyrrole-2,5-dione
| Title | Journal |
|---|---|
| One-electron oxidation of ferrocenes by short-lived N-oxyl radicals. The role of structural effects on the intrinsic electron transfer reactivities. | Organic & biomolecular chemistry 20110607 |
| N-hydroxyimides as efficient ligands for the copper-catalyzed N-arylation of pyrrole, imidazole, and indole. | The Journal of organic chemistry 20071109 |
| Coupling of contact sensitizers to thiol groups is a key event for the activation of monocytes and monocyte-derived dendritic cells. | The Journal of investigative dermatology 20030201 |
| Cyclization strategies for the synthesis of macrocyclic bisindolylmaleimides. | The Journal of organic chemistry 20010323 |