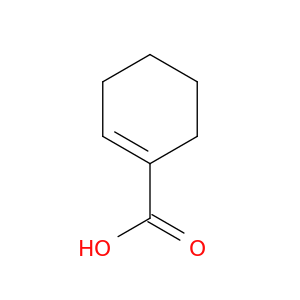
1-Cyclohexene-1-carboxylic acid
| Title | Journal |
|---|---|
| Naphthenic acid biodegradation by the unicellular alga Dunaliella tertiolecta. | Chemosphere 20110701 |
| Physical exercise reduces circulating lipopolysaccharide and TLR4 activation and improves insulin signaling in tissues of DIO rats. | Diabetes 20110301 |
| Biosynthesis of rapamycin and its regulation: past achievements and recent progress. | The Journal of antibiotics 20100801 |
| Factors that affect oxygen activation and coupling of the two redox cycles in the aromatization reaction catalyzed by NikD, an unusual amino acid oxidase. | Biochemistry 20091013 |
| Genomic and microarray analysis of aromatics degradation in Geobacter metallireducens and comparison to a Geobacter isolate from a contaminated field site. | BMC genomics 20070101 |
| Neuroprotective and free radical scavenging activities of phenolic compounds from Hovenia dulcis. | Archives of pharmacal research 20050701 |
| The effects of benzoate, cyclohex-1-ene carboxylate, and cyclohexane carboxylate on biotransformation of o-phthalate in sediment slurries under sulfate-reducing conditions. | Chemosphere 20050301 |
| Degradation pathways of cyclic alkanes in Rhodococcus sp. NDKK48. | Applied microbiology and biotechnology 20041101 |
| Mutasynthesis of enterocin and wailupemycin analogues. | Journal of the American Chemical Society 20030806 |
| High-performance nanocatalysts for single-step hydrogenations. | Accounts of chemical research 20030101 |
| Molecular characterization of NikD, a new flavoenzyme important in the biosynthesis of nikkomycin antibiotics. | Biochemistry 20021231 |