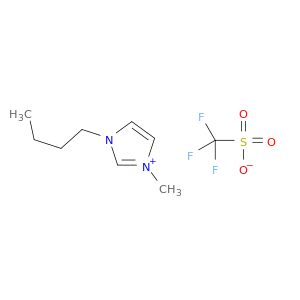
1-Butyl-3-methylimidazolium trifluoromethanesulfonate
| Title | Journal |
|---|---|
| Role of the Hofmeister series in the formation of ionic-liquid-based aqueous biphasic systems. | The journal of physical chemistry. B 20120621 |
| Microwave-assisted separation of ionic liquids from aqueous solution of ionic liquids. | Journal of chromatography. A 20101203 |
| Optimization of lipase-catalyzed glucose ester synthesis in ionic liquids. | Bioprocess and biosystems engineering 20100101 |
| Anion configuration at the air/liquid interface of ionic liquid [bmim]OTf studied by sum-frequency generation spectroscopy. | The journal of physical chemistry. B 20080925 |
| Temperature-programed time-of-flight secondary ion mass spectrometry study of 1-butyl-3-methylimidazolium trifluoromethanesulfonate during glass-liquid transition, crystallization, melting, and solvation. | The Journal of chemical physics 20080907 |