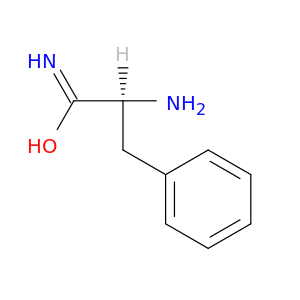
H-L-Phe-NH2
| Title | Journal |
|---|---|
| Antimicrobial chemical constituents from the endophytic fungus Phomopsis sp. from Notobasis syriaca. | Natural product communications 20111201 |
| Biological properties of prolactin-releasing peptide analogs with a modified aromatic ring of a C-terminal phenylalanine amide. | Peptides 20110901 |
| Discovery of dipeptides with high affinity to the specific binding site for substance P1-7. | Journal of medicinal chemistry 20100325 |
| Biochemical properties and potential applications of a solvent-stable protease from the high-yield protease producer Pseudomonas aeruginosa PT121. | Applied biochemistry and biotechnology 20100201 |
| Enhanced cell permeability of kojic acid-phenylalanine amide with metal complex. | Bioorganic & medicinal chemistry letters 20100115 |
| Kojic acid-amino acid conjugates as tyrosinase inhibitors. | Bioorganic & medicinal chemistry letters 20091001 |
| Enantioseparation of dansyl amino acids by ligand-exchange capillary electrophoresis with zinc(II)-L-phenylalaninamide complex. | Journal of separation science 20090901 |
| Synthesis and assessment of molecular recognizability by RP-HPLC of an N-alkyl-beta-Ala-L: -Phe-derived organic phase with self-assembling ability. | Analytical and bioanalytical chemistry 20081101 |
| A selective fluorescence reaction for peptides and chromatographic analysis. | Peptides 20080301 |
| Structures of D-amino-acid amidase complexed with L-phenylalanine and with L-phenylalanine amide: insight into the D-stereospecificity of D-amino-acid amidase from Ochrobactrum anthropi SV3. | Acta crystallographica. Section D, Biological crystallography 20080301 |
| Synthesis, self-assembling properties, and atom transfer radical polymerization of an alkylated L-phenylalanine-derived monomeric organogel from silica: a new approach to prepare packing materials for high-performance liquid chromatography. | Chemistry (Weinheim an der Bergstrasse, Germany) 20080101 |
| Comparative assessment of two indices of drug induced permeability changes in the perfused rat intestine. | International journal of pharmaceutics 20060407 |
| L: -Stereoselective amino acid amidase with broad substrate specificity from Brevundimonas diminuta: characterization of a new member of the leucine aminopeptidase family. | Applied microbiology and biotechnology 20060401 |
| Identification of specific calcitonin-like receptor residues important for calcitonin gene-related peptide high affinity binding. | BMC pharmacology 20060101 |
| Application of Fourier transform infrared spectroscopy for monitoring hydrolysis and synthesis reactions catalyzed by a recombinant amidase. | Analytical biochemistry 20051101 |
| Design of chiral monochloro-s-triazine reagents for the liquid chromatographic separation of amino acid enantiomers. | Journal of chromatography. A 20030523 |
| Ni-to-Ni+ 3-ethylene-bridged partially modified retro-inverso tetrapeptide beta-turn mimetic: design, synthesis, and structural characterization. | The Journal of organic chemistry 20020726 |
| Chemically modified chiral monolithic silica column prepared by a sol-gel process for enantiomeric separation by micro high-performance liquid chromatography. | Journal of chromatography. A 20020104 |
| (R)-3-Amidinophenylalanine-derived inhibitors of factor Xa with a novel active-site binding mode. | Biological chemistry 20020101 |
| In vitro selection of DNA aptamers that bind L-tyrosinamide. | Bioorganic & medicinal chemistry 20011001 |
| Chemically L-phenylalaninamide-modified monolithic silica column prepared by a sol-gel process for enantioseparation of dansyl amino acids by ligand exchange-capillary electrochromatography. | Analytical chemistry 20010715 |