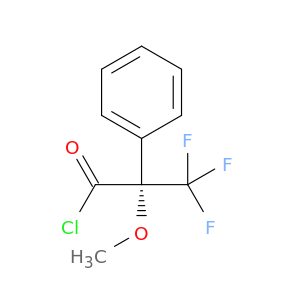
(R)-(+)-α-Methoxy-α-(trifluoromethyl)phenylacetyl chloride
| Title | Journal |
|---|---|
| Stereoselective method development and validation for determination of concentrations of amphetamine-type stimulants and metabolites in human urine using a simultaneous extraction-chiral derivatization approach. | Journal of chromatography. B, Analytical technologies in the biomedical and life sciences 20110101 |
| Development of a simultaneous liquid-liquid extraction and chiral derivatization method for stereospecific GC-MS analysis of amphetamine-type stimulants in human urine using fractional factorial design. | Biomedical chromatography : BMC 20080901 |
| Quantitative and isomeric determination of amphetamine and methamphetamine from urine using a nonprotic elution solvent and R(-)-alpha-methoxy-alpha-trifluoromethylphenylacetic acid chloride derivatization. | Journal of analytical toxicology 20051001 |
| Enantiomeric separation and quantitation of (+/-)-amphetamine, (+/-)-methamphetamine, (+/-)-MDA, (+/-)-MDMA, and (+/-)-MDEA in urine specimens by GC-EI-MS after derivatization with (R)-(-)- or (S)-(+)-alpha-methoxy-alpha-(trifluoromethy)phenylacetyl chloride (MTPA). | Journal of analytical toxicology 20040901 |
| Synthesis, resolution, and determination of the absolute configuration of the enantiomers of cis-4,5-dihydroxy-1,2-dithiane 1,1-dioxide, an HIV-1NCp7 inhibitor. | Bioorganic & medicinal chemistry 20030717 |
| Method development for the high-performance liquid chromatographic enantioseparation of 2-cyanocycloalkanols. | Journal of chromatographic science 20010501 |