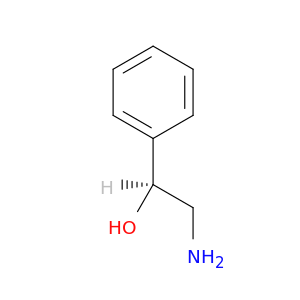
(S)-2-Amino-1-phenylethanol
| Title | Journal |
|---|---|
| On the enantioselectivity of aziridination of styrene catalysed by copper triflate and copper-exchanged zeolite Y: consequences of the phase behaviour of enantiomeric mixtures of N-arene-sulfonyl-2-phenylaziridines. | Organic & biomolecular chemistry 20110221 |
| Enantioselective gel collapsing: a new means of visual chiral sensing. | Journal of the American Chemical Society 20100602 |
| Asymmetric synthesis of 8-aminoindolizidine from chiral 2-pyrroleimines. | The Journal of organic chemistry 20081107 |
| Formal total synthesis of (+)-gephyrotoxin. | The Journal of organic chemistry 20080815 |
| Enantioseparation of phenylglycinol in chiral-modified zeolite HY: a molecular simulation study. | Chirality 20070601 |
| Practical enantioselective synthesis of beta-substituted-beta-amino esters. | The Journal of organic chemistry 20050708 |
| Stereoselective addition of dimethyl thiophosphite to imines. | The Journal of organic chemistry 20040402 |
| Molecular recognition of sub-micromolar inhibitors by the epinephrine-synthesizing enzyme phenylethanolamine N-methyltransferase. | Journal of medicinal chemistry 20040101 |
| Employing the structural diversity of nature: development of modular dipeptide-analogue ligands for ruthenium-catalyzed enantioselective transfer hydrogenation of ketones. | Chemistry (Weinheim an der Bergstrasse, Germany) 20030905 |
| Phenylethanolamine N-methyltransferase kinetics: bovine versus recombinant human enzyme. | Bioorganic & medicinal chemistry letters 20010618 |
| Enantioselective syntheses of dopaminergic (R)- and (S)-benzyltetrahydroisoquinolines. | Journal of medicinal chemistry 20010524 |