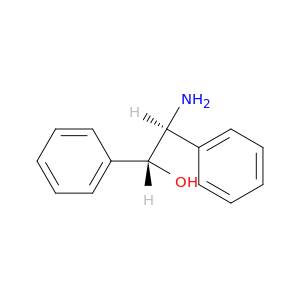
(1R,2S)-(-)-2-Amino-1,2-diphenylethanol
| Title | Journal |
|---|---|
| Synthesis of dendrimer-type chiral stationary phases based on the selector of (1S,2R)-(+)-2-amino-1,2-diphenylethanol derivate and their enantioseparation evaluation by HPLC. | Chirality 20100101 |
| Beta-amino alcohol derived beta-hydroxy- and beta-(o-diphenylphosphino)benzoyloxy(o-diphenylphosphino)benzamides: an ester-amide ligand structural model for the palladium-catalyzed allylic alkylation reaction. | The Journal of organic chemistry 20091106 |
| NMDA receptor affinities of 1,2-diphenylethylamine and 1-(1,2-diphenylethyl)piperidine enantiomers and of related compounds. | Bioorganic & medicinal chemistry 20090501 |
| Immobilization of (1S,2R)-(+)-2-amino-1,2-diphenylethanol derivates on aminated silica gel with different linkages as chiral stationary phases and their enantioseparation evaluation by HPLC. | Chirality 20090401 |
| A solid-state fluorescent host system with a 2(1)-helical column consisting of chiral (1R,2S)-2-amino-1,2-diphenylethanol and fluorescent 1-pyrenecarboxylic acid. | Chemistry, an Asian journal 20080307 |
| A solid-state fluorescence sensing system consisting of chiral (1R,2S)-2-amino-1,2-diphenylethanol and fluorescent 2-anthracenecarboxylic acid. | Organic letters 20070816 |
| Synthesis of C3-symmetric tris(beta-hydroxy amide) ligands and their Ti(IV) complex-catalyzed enantioselective alkynylation of aldehydes. | Organic letters 20050526 |
| Efficient crystallization-induced dynamic resolution of alpha-substituted carboxylic acids. | The Journal of organic chemistry 20040611 |
| Modification of (1R,2S)-1,2-diphenyl-2-aminoethanol for the highly enantioselective, asymmetric alkylation of N-diphenylphosphinoyl arylimines with dialkylzinc. | Chemistry (Weinheim an der Bergstrasse, Germany) 20040319 |
| An amino alcohol ligand for highly enantioselective addition of organozinc reagents to aldehydes: serendipity rules. | Organic letters 20020627 |
| Asymmetric synthesis of [2,3-(13)C(2),(15)N]-4-benzyloxy-5,6-diphenyl-2,3,5,6-tetrahydro-4H-oxazine-2-one via lipase TL-mediated kinetic resolution of benzoin: general procedure for the synthesis of [2,3-(13)C(2),(15)N]-L-alanine. | The Journal of organic chemistry 20011130 |