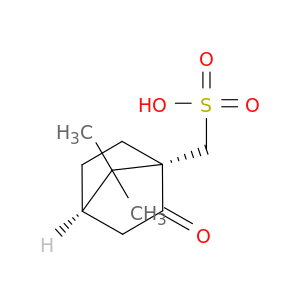
(1S)-(+)-10-Camphorsulfonic acid
| Title | Journal |
|---|---|
| Camphorsulfonic acid catalysed facile tandem double Friedlander annulation protocol for the synthesis of phenoxy linked bisquinoline derivatives and discovery of antitubercular agents. | Bioorganic & medicinal chemistry letters 20120215 |
| Primary amine/CSA ion pair: a powerful catalytic system for the asymmetric enamine catalysis. | Organic letters 20110520 |
| Determination of bile acids in pig liver, pig kidney and bovine liver by gas chromatography-chemical ionization tandem mass spectrometry with total ion chromatograms and extraction ion chromatograms. | Journal of chromatography. A 20110121 |
| Analysis of deprotonated acids with silicon nanoparticle-assisted laser desorption/ ionization mass spectrometry. | Journal of mass spectrometry : JMS 20101201 |
| Design and construction of a compact end-station at NSRRC for circular-dichroism spectra in the vacuum-ultraviolet region. | Journal of synchrotron radiation 20101101 |
| Primary amine/(+)-CSA salt-promoted organocatalytic conjugate addition of nitro esters to enones. | Organic letters 20100521 |
| Swift heavy ion irradiation induced enhancement in the antioxidant activity and biocompatibility of polyaniline nanofibers. | Nanotechnology 20100430 |
| (S)-Camphorsulfonic acid catalyzed highly stereoselective synthesis of pseudoglycosides. | Bioorganic & medicinal chemistry letters 20090601 |
| Solute-solvent interactions in imidazolium camphorsulfonate ionic liquids. | Physical chemistry chemical physics : PCCP 20071128 |
| Micellar peroxidase-catalyzed synthesis of chiral polyaniline. | Biomacromolecules 20070801 |
| Effect of (+) or (-) camphorsulfonic acid additives to the mobile phase on enantioseparations of some basic drugs on a Chiralcel OD column. | Journal of chromatography. A 20050812 |
| Redetermination of the extinction coefficient of camphor-10-sulfonic acid, a calibration standard for circular dichroism spectroscopy. | Analytical biochemistry 20041215 |
| Enantiomeric separation of basic compounds using heptakis(2,3-di-O-methyl-6-O-sulfo)-beta-cyclodextrin in combination with potassium camphorsulfonate in nonaqueous capillary electrophoresis: optimization by means of an experimental design. | Electrophoresis 20040801 |
| Transglucosidation of methyl and ethyl D-glucopyranosides by alcoholysis. | Carbohydrate research 20020315 |