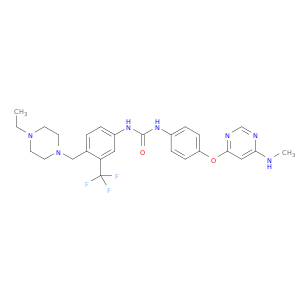
1-(4-((4-Ethylpiperazin-1-yl)methyl)-3-(trifluoromethyl)phenyl)-3-(4-((6-(methylamino)pyrimidin-4-yl)oxy)phenyl)urea
| Title | Journal |
|---|---|
| Comprehensive analysis of kinase inhibitor selectivity. | Nature biotechnology 20111030 |
| Discovery, synthesis, and investigation of the antitumor activity of novel piperazinylpyrimidine derivatives. | European journal of medicinal chemistry 20110601 |
| Activation state-dependent binding of small molecule kinase inhibitors: structural insights from biochemistry. | Chemistry & biology 20101124 |
| Antileukemic effects of the novel, mutant FLT3 inhibitor NVP-AST487: effects on PKC412-sensitive and -resistant FLT3-expressing cells. | Blood 20081215 |
| A quantitative analysis of kinase inhibitor selectivity. | Nature biotechnology 20080101 |
| The RET kinase inhibitor NVP-AST487 blocks growth and calcitonin gene expression through distinct mechanisms in medullary thyroid cancer cells. | Cancer research 20070715 |