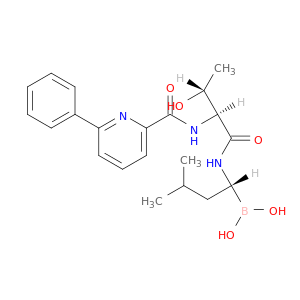
[(1R)-1-[(2S,3R)-3-hydroxy-2-[(6-phenylpyridin-2-yl)formamido]butanamido]-3-methylbutyl]boronic acid
| Title | Journal |
|---|---|
| Molecular mechanisms of acquired proteasome inhibitor resistance. | Journal of medicinal chemistry 20121213 |
| CEP-18770 (delanzomib) in combination with dexamethasone and lenalidomide inhibits the growth of multiple myeloma. | Leukemia research 20121101 |
| Novel, orally active, proteasome inhibitor, delanzomib (CEP-18770), ameliorates disease symptoms and glomerulonephritis in two preclinical mouse models of SLE. | International immunopharmacology 20120101 |
| Analysing properties of proteasome inhibitors using kinetic and X-ray crystallographic studies. | Methods in molecular biology (Clifton, N.J.) 20120101 |
| Drugs: More shots on target. | Nature 20111214 |
| Novel proteasome inhibitors to overcome bortezomib resistance. | Journal of the National Cancer Institute 20110706 |
| Development and validation of a high-performance liquid chromatography-tandem mass spectrometry method for the determination of the novel proteasome inhibitor CEP-18770 in human plasma and its application in a clinical pharmacokinetic study. | Journal of mass spectrometry : JMS 20101101 |
| Toward the development of innovative bifunctional agents to induce differentiation and to promote apoptosis in leukemia: clinical candidates and perspectives. | Journal of medicinal chemistry 20101014 |
| The proteasome inhibitor CEP-18770 enhances the anti-myeloma activity of bortezomib and melphalan. | British journal of haematology 20100201 |
| CEP-18770: A novel, orally active proteasome inhibitor with a tumor-selective pharmacologic profile competitive with bortezomib. | Blood 20080301 |
| Discovery of a potent, selective, and orally active proteasome inhibitor for the treatment of cancer. | Journal of medicinal chemistry 20080227 |