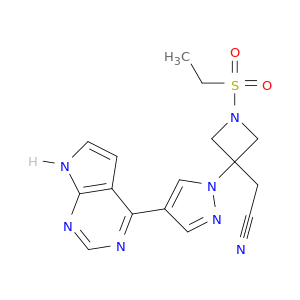
2-(3-(4-(7H-Pyrrolo[2,3-d]pyrimidin-4-yl)-1H-pyrazol-1-yl)-1-(ethylsulfonyl)azetidin-3-yl)acetonitrile
| Title | Journal |
|---|---|
| Baricitinib in Patients with Refractory Rheumatoid Arthritis. | The New England journal of medicine 20160331 |
| Reversal of Alopecia Areata Following Treatment With the JAK1/2 Inhibitor Baricitinib. | EBioMedicine 20150401 |
| Alopecia areata is driven by cytotoxic T lymphocytes and is reversed by JAK inhibition. | Nature medicine 20140901 |
| Discovery and development of Janus kinase (JAK) inhibitors for inflammatory diseases. | Journal of medicinal chemistry 20140626 |
| Inhibition of Janus kinase/signal transducer and activator of transcription (JAK/STAT) signalling pathway in rheumatoid synovial fibroblasts using small molecule compounds. | Clinical and experimental immunology 20131201 |
| Selective inhibition of JAK1 and JAK2 is efficacious in rodent models of arthritis: preclinical characterization of INCB028050. | Journal of immunology (Baltimore, Md. : 1950) 20100501 |