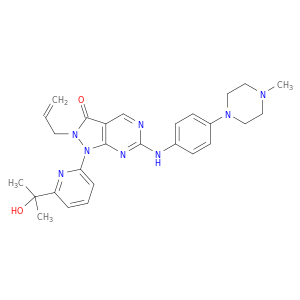
1-[6-(2-hydroxypropan-2-yl)pyridin-2-yl]-6-{[4-(4-methylpiperazin-1-yl)phenyl]amino}-2-(prop-2-en-1-yl)pyrazolo[3,4-d]pyrimidin-3-one
| Title | Journal |
|---|---|
| Functional kinomics identifies candidate therapeutic targets in head and neck cancer. | Clinical cancer research : an official journal of the American Association for Cancer Research 20140815 |
| Identification of potent Yes1 kinase inhibitors using a library screening approach. | Bioorganic & medicinal chemistry letters 20130801 |
| Forced mitotic entry of S-phase cells as a therapeutic strategy induced by inhibition of WEE1. | Cancer discovery 20120601 |
| MK1775, a selective Wee1 inhibitor, shows single-agent antitumor activity against sarcoma cells. | Molecular cancer therapeutics 20120101 |
| MK-1775, a potent Wee1 inhibitor, synergizes with gemcitabine to achieve tumor regressions, selectively in p53-deficient pancreatic cancer xenografts. | Clinical cancer research : an official journal of the American Association for Cancer Research 20110501 |
| Targeting Wee1-like protein kinase to treat cancer. | Drug news & perspectives 20100901 |
| Abrogation of the G2 checkpoint by inhibition of Wee-1 kinase results in sensitization of p53-deficient tumor cells to DNA-damaging agents. | Current clinical pharmacology 20100801 |
| MK-1775, a small molecule Wee1 inhibitor, enhances anti-tumor efficacy of various DNA-damaging agents, including 5-fluorouracil. | Cancer biology & therapy 20100401 |
| Small-molecule inhibition of Wee1 kinase by MK-1775 selectively sensitizes p53-deficient tumor cells to DNA-damaging agents. | Molecular cancer therapeutics 20091101 |