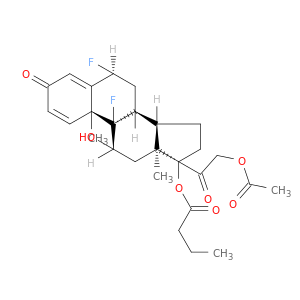
(6α,11β)-21-(Acetyloxy)-6,9-difluoro-11-hydroxy-17-(1-oxobutoxy)pregna-1,4-diene-3,20-dion
| Title | Journal |
|---|---|
| Treatment of diffuse diabetic macular oedema using steroid eye drops. | Acta ophthalmologica 20121101 |
| Hypopyon uveitis following LASIK in a patient with ulcerative colitis. | Journal of refractive surgery (Thorofare, N.J. : 1995) 20120801 |
| Efficacy and potential complications of difluprednate use for pediatric uveitis. | American journal of ophthalmology 20120501 |
| Vogt-Koyanagi-Harada syndrome in a 6-year-old Hispanic boy. | Pediatric dermatology 20120101 |
| Graft rejection after Descemet's stripping automated endothelial keratoplasty: graft survival and endothelial cell loss. | Ophthalmology 20120101 |
| Chronic anterior uveitis in common variable immunodeficiency. | Ocular immunology and inflammation 20111201 |
| A multicenter randomized controlled fellow eye trial of pulse-dosed difluprednate 0.05% versus prednisolone acetate 1% in cataract surgery. | American journal of ophthalmology 20111001 |
| Elevation of intraocular pressure in patients with uveitis treated with topical difluprednate. | Archives of ophthalmology (Chicago, Ill. : 1960) 20110501 |
| Difluprednate for inflammatory eye disorders. | Drugs of today (Barcelona, Spain : 1998) 20110501 |
| Effects of twice-daily topical difluprednate 0.05% emulsion in a child with pars planitis. | Ocular immunology and inflammation 20110201 |
| Ocular distribution of difluprednate ophthalmic emulsion 0.05% in rabbits. | Journal of ocular pharmacology and therapeutics : the official journal of the Association for Ocular Pharmacology and Therapeutics 20110201 |
| Pharmacokinetic features of difluprednate ophthalmic emulsion in rabbits as determined by glucocorticoid receptor-binding bioassay. | Journal of ocular pharmacology and therapeutics : the official journal of the Association for Ocular Pharmacology and Therapeutics 20110201 |
| Intraocular pressure elevation from topical difluprednate use. | Optometry (St. Louis, Mo.) 20101201 |
| Durezol (Difluprednate Ophthalmic Emulsion 0.05%) compared with Pred Forte 1% ophthalmic suspension in the treatment of endogenous anterior uveitis. | Journal of ocular pharmacology and therapeutics : the official journal of the Association for Ocular Pharmacology and Therapeutics 20101001 |
| Metabolic profiles of difluprednate in rabbit ocular tissues after instillation of difluprednate ophthalmic emulsion. | Xenobiotica; the fate of foreign compounds in biological systems 20100801 |
| Steroid eye drop treatment (difluprednate ophthalmic emulsion) is effective in reducing refractory diabetic macular edema. | Graefe's archive for clinical and experimental ophthalmology = Albrecht von Graefes Archiv fur klinische und experimentelle Ophthalmologie 20100601 |
| New drugs 09, part 2. | Nursing 20090601 |
| Difluprednate ophthalmic emulsion 0.05% for postoperative inflammation and pain. | Journal of cataract and refractive surgery 20090101 |
| The role of difluprednate ophthalmic emulsion in clinical practice. | Clinical ophthalmology (Auckland, N.Z.) 20090101 |
| New drugs: Clevidipine butyrate, difluprednate, and tetrabenazine. | Journal of the American Pharmacists Association : JAPhA 20080101 |
| Microsphere-based protease assays and screening application for lethal factor and factor Xa. | Cytometry. Part A : the journal of the International Society for Analytical Cytology 20060501 |
| Injection site with generalized rash caused by pegylated interferon alpha 2a injection. | Dermatology (Basel, Switzerland) 20060101 |
| Formulation of an ophthalmic lipid emulsion containing an anti-inflammatory steroidal drug, difluprednate. | International journal of pharmaceutics 20050914 |
| Cutaneous reactions to imatinib mesylate treated by topical steroid. | American journal of hematology 20050301 |
| Analysis of an anti-inflammatory steroidal drug, difluprednate, in aqueous humor by combination of semi-micro HPLC and column switching method. | Journal of pharmaceutical and biomedical analysis 20030115 |
| Neuronal cell bodies in the hypothalamic paraventricular nucleus mediate stress-induced renin and corticosterone secretion. | Neuroendocrinology 19890701 |