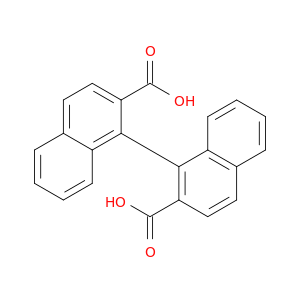
S-1,1'-binaphthyl-2,2'-dicarboxylic Acid
| Title | Journal |
|---|---|
| An allosteric conduit facilitates dynamic multisite substrate recognition by the SCF(Cdc4) ubiquitin ligase. | Nature communications 20170101 |
| Generation and exploitation of acyclic azomethine imines in chiral Brønsted acid catalysis. | Nature chemistry 20110722 |
| An allosteric inhibitor of substrate recognition by the SCF(Cdc4) ubiquitin ligase. | Nature biotechnology 20100701 |
| 1,1'-Binaphthyl-2,2'-dicarboxylic acid-urea (1/1). | Acta crystallographica. Section E, Structure reports online 20081001 |
| Combinatorial design of simplified high-performance chiral phase-transfer catalysts for practical asymmetric synthesis of alpha-alkyl- and alpha,alpha-dialkyl-alpha-amino acids. | Chemistry, an Asian journal 20080901 |
| Tuning mechanism in a two-component columnar host system composed of 1,2-diphenylethylenediamine and 1,1'-binaphthyl-2,2'-dicarboxylic acid. | Organic letters 20080207 |
| A coincident spontaneous resolution system for racemic 1,1'-binaphthyl-2,2'-dicarboxylic acid and 1,2-diphenylethylenediamine induced by water. | Chemical communications (Cambridge, England) 20080121 |
| Two-dimensional supramolecular arrangements of enantiomers and racemic modification of 1,1'-binaphthyl-2,2'-dicarboxylic acid. | Langmuir : the ACS journal of surfaces and colloids 20050927 |