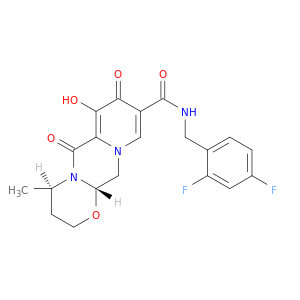
(3S,7R)-N-[(2,4-difluorophenyl)methyl]-11-hydroxy-7-methyl-9,12-dioxo-4-oxa-1,8-diazatricyclo[8.4.0.0^{3,8}]tetradeca-10,13-diene-13-carboxamide
| Title | Journal |
|---|---|
| Antiviral Activity of Bictegravir and Cabotegravir against Integrase Inhibitor-Resistant SIVmac239 and HIV-1. | Antimicrobial agents and chemotherapy 20171201 |
| Impact of HIV-1 Integrase L74F and V75I Mutations in a Clinical Isolate on Resistance to Second-Generation Integrase Strand Transfer Inhibitors. | Antimicrobial agents and chemotherapy 20170801 |
| Antiviral characteristics of GSK1265744, an HIV integrase inhibitor dosed orally or by long-acting injection. | Antimicrobial agents and chemotherapy 20150101 |
| Clinical pharmacokinetic, pharmacodynamic and drug-interaction profile of the integrase inhibitor dolutegravir. | Clinical pharmacokinetics 20131101 |
| Dolutegravir (Tivicay) for HIV. | The Medical letter on drugs and therapeutics 20130930 |
| Dolutegravir: first global approval. | Drugs 20130901 |
| Dolutegravir versus raltegravir in antiretroviral-experienced, integrase-inhibitor-naive adults with HIV: week 48 results from the randomised, double-blind, non-inferiority SAILING study. | Lancet (London, England) 20130824 |
| Antiretroviral therapy: dolutegravir sets SAIL(ING). | Lancet (London, England) 20130824 |
| Impact of primary elvitegravir resistance-associated mutations in HIV-1 integrase on drug susceptibility and viral replication fitness. | Antimicrobial agents and chemotherapy 20130601 |
| In vitro investigations into the roles of drug transporters and metabolizing enzymes in the disposition and drug interactions of dolutegravir, a HIV integrase inhibitor. | Drug metabolism and disposition: the biological fate of chemicals 20130201 |
| The activity of the integrase inhibitor dolutegravir against HIV-1 variants isolated from raltegravir-treated adults. | Journal of acquired immune deficiency syndromes (1999) 20121101 |
| Prevalence of HIV-1 integrase mutations related to resistance to dolutegravir in raltegravir naïve and pretreated patients. | Clinical microbiology and infection : the official publication of the European Society of Clinical Microbiology and Infectious Diseases 20121001 |
| The use of HIV-1 integrase inhibitors in antiretroviral naive patients. | Current opinion in HIV and AIDS 20120901 |
| Pharmacology of HIV integrase inhibitors. | Current opinion in HIV and AIDS 20120901 |
| HIV integrase inhibitors in ART-experienced patients. | Current opinion in HIV and AIDS 20120901 |
| Tolerability of HIV integrase inhibitors. | Current opinion in HIV and AIDS 20120901 |
| From in vitro EC₅₀ to in vivo dose-response for antiretrovirals using an HIV disease model. Part I: a framework. | Journal of pharmacokinetics and pharmacodynamics 20120801 |
| Update on raltegravir and the development of new integrase strand transfer inhibitors. | Southern medical journal 20120701 |
| Dolutegravir for the treatment of HIV. | Expert opinion on investigational drugs 20120401 |
| Effect of a single supratherapeutic dose of dolutegravir on cardiac repolarization. | Pharmacotherapy 20120401 |
| Once daily dolutegravir (S/GSK1349572) in combination therapy in antiretroviral-naive adults with HIV: planned interim 48 week results from SPRING-1, a dose-ranging, randomised, phase 2b trial. | The Lancet. Infectious diseases 20120201 |
| Genetic barrier to the development of resistance to integrase inhibitors in HIV-1 subtypes CRF01_AE and B. | Intervirology 20120101 |
| Cross-resistance profile of the novel integrase inhibitor Dolutegravir (S/GSK1349572) using clonal viral variants selected in patients failing raltegravir. | The Journal of infectious diseases 20111201 |
| Structural and functional analyses of the second-generation integrase strand transfer inhibitor dolutegravir (S/GSK1349572). | Molecular pharmacology 20111001 |
| Antiviral activity, safety, and pharmacokinetics/pharmacodynamics of dolutegravir as 10-day monotherapy in HIV-1-infected adults. | AIDS (London, England) 20110910 |
| Effect of atazanavir and atazanavir/ritonavir on the pharmacokinetics of the next-generation HIV integrase inhibitor, S/GSK1349572. | British journal of clinical pharmacology 20110701 |
| Prevalence of resistance mutations related to integrase inhibitor S/GSK1349572 in HIV-1 subtype B raltegravir-naive and -treated patients. | The Journal of antimicrobial chemotherapy 20110701 |
| Pharmacokinetics of the HIV integrase inhibitor S/GSK1349572 co-administered with acid-reducing agents and multivitamins in healthy volunteers. | The Journal of antimicrobial chemotherapy 20110701 |
| S/GSK1349572, a new integrase inhibitor for the treatment of HIV: promises and challenges. | Expert opinion on investigational drugs 20110401 |
| Implications of integrase inhibitors for HIV-infected transplantation recipients: raltegravir and dolutegravir (S/GSK 1349572). | Bioscience trends 20110101 |
| Pharmacokinetics and safety of S/GSK1349572, a next-generation HIV integrase inhibitor, in healthy volunteers. | Antimicrobial agents and chemotherapy 20100101 |