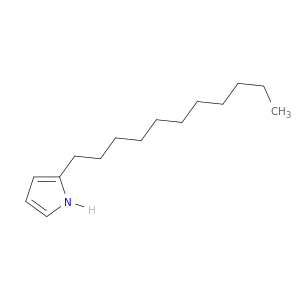
1H-Pyrrole, 2-undecyl-
| Title | Journal |
|---|---|
| Stereochemical elucidation of streptorubin B. | Journal of the American Chemical Society 20110216 |
| Role and substrate specificity of the Streptomyces coelicolor RedH enzyme in undecylprodiginine biosynthesis. | Chemical communications (Cambridge, England) 20080428 |
| Elucidation of the Streptomyces coelicolor pathway to 2-undecylpyrrole, a key intermediate in undecylprodiginine and streptorubin B biosynthesis. | Chemistry & biology 20080201 |
| Biosynthesis of the red antibiotic, prodigiosin, in Serratia: identification of a novel 2-methyl-3-n-amyl-pyrrole (MAP) assembly pathway, definition of the terminal condensing enzyme, and implications for undecylprodigiosin biosynthesis in Streptomyces. | Molecular microbiology 20050501 |