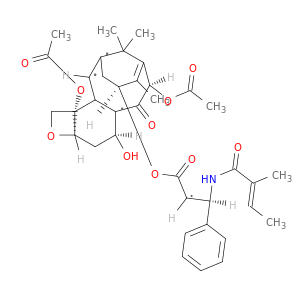
Benzenepropanoic acid, α-hydroxy-β-[[(2E)-2-methyl-1-oxo-2-buten-1-yl]amino]-, (2aR,4S,4aS,6R,9S,11S,12S,12aR,12bS)-6,12b-bis(acetyloxy)-12-(benzoyloxy)-2a,3,4,4a,5,6,9,10,11,12,12a,12b-dodecahydro-4,11-dihydroxy-4a,8,13,13-tetramethyl-5-oxo-7,11-methano-1H-cyclodeca[3,4]benz[1,2-b]oxet-9-yl ester, (αR,βS)-
| Title | Journal |
|---|---|
| Fatal poisoning with Taxus baccata: quantification of paclitaxel (taxol A), 10-deacetyltaxol, baccatin III, 10-deacetylbaccatin III, cephalomannine (taxol B), and 3,5-dimethoxyphenol in body fluids by liquid chromatography-tandem mass spectrometry. | Journal of analytical toxicology 20120101 |
| C-7 configuration as one of determinants in taxanes metabolism by human cytochrome P450 enzymes. | Xenobiotica; the fate of foreign compounds in biological systems 20091201 |
| On the origins of the universal dynamics of endogenous granules in mammalian cells. | Molecular & cellular biomechanics : MCB 20091201 |
| Histone deacetylase and microtubules as targets for the synthesis of releasable conjugate compounds. | Bioorganic & medicinal chemistry letters 20091115 |
| Preparative separation and enrichment of four taxoids from Taxus chinensis needles extracts by macroporous resin column chromatography. | Journal of separation science 20090501 |
| Synthesis and biological activities of high affinity taxane-based fluorescent probes. | Bioorganic & medicinal chemistry letters 20090201 |
| Overcoming tumor drug resistance with C2-modified 10-deacetyl-7-propionyl cephalomannines: a QSAR study. | Molecular pharmaceutics 20090101 |
| Lx2-32c, a novel taxane and its antitumor activities in vitro and in vivo. | Cancer letters 20080908 |
| [Quantitative changes of anti-cancer active components in Taxus chinensis var. mairei branches and leaves]. | Ying yong sheng tai xue bao = The journal of applied ecology 20080401 |
| Taxane's substituents at C3' affect its regioselective metabolism: different in vitro metabolism of cephalomannine and paclitaxel. | Drug metabolism and disposition: the biological fate of chemicals 20080201 |
| Microbial transformation of cephalomannine by Luteibacter sp. | Journal of natural products 20071201 |
| Rapid separation of four main taxoids in Taxus species by a combined LLP-SPE-HPLC (PAD) procedure. | Journal of separation science 20060601 |
| A novel bioassay for screening and quantification of taxanes. | Chemical communications (Cambridge, England) 20030521 |
| New taxane analogues from the needles of Taxus canadensis. | Journal of natural products 20010401 |