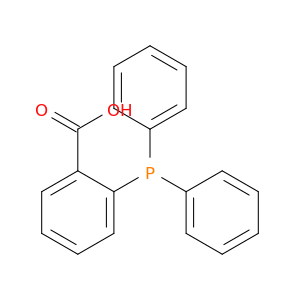
2-(Diphenylphosphino)benzoic acid
| Title | Journal |
|---|---|
| Stereoselective synthesis of trisubstituted olefins by a directed allylic substitution strategy. | Chemistry (Weinheim an der Bergstrasse, Germany) 20111010 |
| Stereoselective and diversity-oriented synthesis of trisubstituted allylic alcohols and amines. | Chemistry (Weinheim an der Bergstrasse, Germany) 20111010 |
| Ruthenium-catalysed linear-selective allylic alkylation of allyl acetates. | Chemical communications (Cambridge, England) 20070121 |
| Electronic differentiation competes with transition state sensitivity in palladium-catalyzed allylic substitutions. | Beilstein journal of organic chemistry 20070101 |
| Predicting the stereochemistry of diphenylphosphino benzoic acid (DPPBA)-based palladium-catalyzed asymmetric allylic alkylation reactions: a working model. | Accounts of chemical research 20061001 |
| Hybridization dependent cleavage of internally modified disulfide-peptide nucleic acids. | Bioorganic & medicinal chemistry letters 20050201 |
| Synthesis, characterization and antioxidant activity of new copper(I) complexes of scorpionate and water soluble phosphane ligands. | Dalton transactions (Cambridge, England : 2003) 20040907 |
| Heterodimerization of olefins. 1. Hydrovinylation reactions of olefins that are amenable to asymmetric catalysis. | The Journal of organic chemistry 20031031 |
| Dynamic kinetic asymmetric cycloadditions of isocyanates to vinylaziridines. | Journal of the American Chemical Society 20031001 |
| New diphosphine ligands containing ethyleneglycol and amino alcohol spacers for the rhodium-catalyzed carbonylation of methanol. | Chemistry (Weinheim an der Bergstrasse, Germany) 20020802 |
| Geminal dicarboxylates as carbonyl surrogates for asymmetric synthesis. Part I. Asymmetric addition of malonate nucleophiles. | Journal of the American Chemical Society 20010425 |