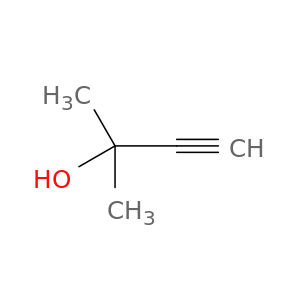
2-Methyl-3-butyn-2-ol
| Title | Journal |
|---|---|
| 5,8-Bis(3-hy-droxy-3-methyl-but-1-yn-1-yl)-2,11-dithia-[3.3]paracyclo-phane. | Acta crystallographica. Section E, Structure reports online 20111201 |
| A computational study on the role of chiral N-oxides in enantioselective Pauson-Khand reactions. | Chemistry (Weinheim an der Bergstrasse, Germany) 20110829 |
| Structure sensitivity of alkynol hydrogenation on shape- and size-controlled palladium nanocrystals: which sites are most active and selective? | Journal of the American Chemical Society 20110817 |
| Basic reactivity of CaO: investigating active sites under operating conditions. | Physical chemistry chemical physics : PCCP 20101128 |
| 4,4'-[2,5-Bis(dodec-yloxy)-p-phenyl-ene]bis-(2-methyl-but-3-yn-2-ol). | Acta crystallographica. Section E, Structure reports online 20100701 |
| 4-(4-Meth-oxy-phen-yl)-2-methyl-but-3-yn-2-ol. | Acta crystallographica. Section E, Structure reports online 20100701 |
| 9-Isopropenyl-4-methyl-2H-thieno[2,3-h]chromen-2-one. | Acta crystallographica. Section E, Structure reports online 20090601 |
| 2,2'-Dimethyl-4,4'-(sulfonyldi-p-phenyl-ene)dibut-3-yn-2-ol dihydrate. | Acta crystallographica. Section E, Structure reports online 20090201 |
| 4-(9-Anthryl)-2-methylbutyn-2-ol. | Acta crystallographica. Section E, Structure reports online 20080201 |
| Ligand accelerated indium(III)-catalyzed asymmetric alkynylation of aldehydes with 2-methyl-3-butyn-2-ol as an ethyne equivalent donor. | Chemical communications (Cambridge, England) 20070307 |
| Hydride-alkenylcarbyne to alkenylcarbene transformation in bisphosphine-osmium complexes. | Journal of the American Chemical Society 20050810 |
| Tandem Sonogashira coupling: an efficient tool for the synthesis of diarylalkynes. | Organic letters 20041223 |
| Six- and eightfold palladium-catalyzed cross-coupling reactions of hexa- and octabromoarenes. | Chemistry (Weinheim an der Bergstrasse, Germany) 20041217 |
| Regioselective synthesis of 6-alkyl- and 6-prenylpolyhydroxyisoflavones and 6-alkylcoumaronochromone derivatives. | Chemical & pharmaceutical bulletin 20041101 |
| Synthesis, X-ray crystal structure and biological properties of acetylenic flavone derivatives. | Farmaco (Societa chimica italiana : 1989) 20030901 |
| One step Pd(0)-catalyzed synthesis, X-ray analysis, and photophysical properties of cyclopent[hi]aceanthrylene: fullerene-like properties in a nonalternant cyclopentafused aromatic hydrocarbon. | Journal of the American Chemical Society 20020109 |