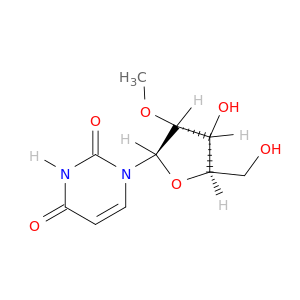
1-((2R,3R,4R,5R)-4-Hydroxy-5-(hydroxymethyl)-3-methoxytetrahydrofuran-2-yl)pyrimidine-2,4(1H,3H)-dione
| Title | Journal |
|---|---|
| Synthesis, gene-silencing activity and nuclease resistance of 3'-3'-linked double short hairpin RNA. | Bioorganic & medicinal chemistry 20101201 |
| Transfer RNA modifications and genes for modifying enzymes in Arabidopsis thaliana. | BMC plant biology 20100101 |
| Chemical synthesis of LNA-2-thiouridine and its influence on stability and selectivity of oligonucleotide binding to RNA. | Biochemistry 20091124 |
| Effect of a water molecule on the sugar puckering of uridine, 2'-deoxyuridine, and 2'-O-methyl uridine inserted in duplexes. | The journal of physical chemistry. A 20080207 |
| Suppression of immunostimulatory siRNA-driven innate immune activation by 2'-modified RNAs. | Biochemical and biophysical research communications 20070914 |
| Synthesis and in vitro anti-mycobacterial activity of 5-substituted pyrimidine nucleosides. | Bioorganic & medicinal chemistry 20051215 |
| Design and studies of novel 5-substituted alkynylpyrimidine nucleosides as potent inhibitors of mycobacteria. | Journal of medicinal chemistry 20051103 |
| A specific substrate-inhibitor, a 2'-deoxy-2'-fluorouridine-containing oligoribonucleotide, against human RNase L. | Bioorganic & medicinal chemistry 20031117 |
| 2'-O-[2-(methylthio)ethyl]-modified oligonucleotide: an analogue of 2'-O-[2-(methoxy)-ethyl]-modified oligonucleotide with improved protein binding properties and high binding affinity to target RNA. | Biochemistry 20021001 |