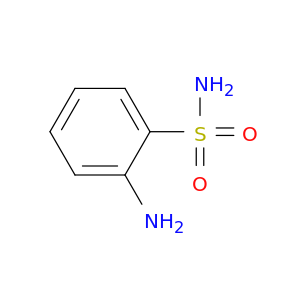
2-Aminobenzenesulfonamide
| Title | Journal |
|---|---|
| Molecular cloning, characterization, and inhibition studies of a β-carbonic anhydrase from Malassezia globosa, a potential antidandruff target. | Journal of medicinal chemistry 20120412 |
| Cloning, characterization and sulfonamide inhibition studies of an α-carbonic anhydrase from the living fossil sponge Astrosclera willeyana. | Bioorganic & medicinal chemistry 20120215 |
| Label-free detection of protein binding with multisine SPR microchips. | Lab on a chip 20111221 |
| Inhibition studies of the β-carbonic anhydrases from the bacterial pathogen Salmonella enterica serovar Typhimurium with sulfonamides and sulfamates. | Bioorganic & medicinal chemistry 20110815 |
| A new β-carbonic anhydrase from Brucella suis, its cloning, characterization, and inhibition with sulfonamides and sulfamates, leading to impaired pathogen growth. | Bioorganic & medicinal chemistry 20110201 |
| Carbonic anhydrase inhibitors. Inhibition studies with anions and sulfonamides of a new cytosolic enzyme from the scleractinian coral Stylophora pistillata. | Bioorganic & medicinal chemistry letters 20110115 |
| 3-(6-Bromo-4-oxo-4H-chromen-3-yl)-3,4-dihydro-2H-1,2,4-benzothia-diazine-1,1-dione. | Acta crystallographica. Section E, Structure reports online 20101201 |
| Synthesis and SAR studies of 1,4-benzoxazine MenB inhibitors: novel antibacterial agents against Mycobacterium tuberculosis. | Bioorganic & medicinal chemistry letters 20101101 |
| 3-(6-Fluoro-4-oxo-4H-chromen-3-yl)-3,4-dihydro-2H-1,2,4-benzothia-diazine-1,1-dione. | Acta crystallographica. Section E, Structure reports online 20101101 |
| Syntheses and evaluation of anticonvulsant profile and teratogenicity of novel amide derivatives of branched aliphatic carboxylic acids with 4-aminobenzensulfonamide. | Journal of medicinal chemistry 20100527 |
| 3D-QSAR study of benzene sulfonamide analogs as carbonic anhydrase II inhibitors. | Bioorganic & medicinal chemistry letters 20100515 |
| Cloning, characterization, and inhibition studies of a beta-carbonic anhydrase from Brucella suis. | Journal of medicinal chemistry 20100311 |
| Gene expression signature-based screening identifies new broadly effective influenza a antivirals. | PloS one 20100101 |
| Carbonic anhydrase inhibitors. Characterization and inhibition studies of the most active beta-carbonic anhydrase from Mycobacterium tuberculosis, Rv3588c. | Bioorganic & medicinal chemistry letters 20091201 |
| Carbonic anhydrase inhibitors. Inhibition studies of a coral secretory isoform by sulfonamides. | Bioorganic & medicinal chemistry 20090715 |
| Carbonic anhydrase inhibitors. Inhibition and homology modeling studies of the fungal beta-carbonic anhydrase from Candida albicans with sulfonamides. | Bioorganic & medicinal chemistry 20090701 |
| Carbonic anhydrase inhibitors. Cloning, characterization, and inhibition studies of a new beta-carbonic anhydrase from Mycobacterium tuberculosis. | Journal of medicinal chemistry 20090514 |
| Molecular cloning, characterization, and inhibition studies of the Rv1284 beta-carbonic anhydrase from Mycobacterium tuberculosis with sulfonamides and a sulfamate. | Journal of medicinal chemistry 20090423 |
| A novel class of allosteric modulators of AMPA/Kainate receptors. | Bioorganic & medicinal chemistry letters 20090215 |
| Carbonic anhydrase inhibitors: inhibition of the beta-class enzyme from the yeast Saccharomyces cerevisiae with sulfonamides and sulfamates. | Bioorganic & medicinal chemistry 20090201 |
| Hepatitis C virus NS3 protease inhibitors comprising a novel aromatic P1 moiety. | Bioorganic & medicinal chemistry 20080315 |
| Carbonic anhydrase inhibitors: cloning, characterization, and inhibition studies of the cytosolic isozyme III with sulfonamides. | Bioorganic & medicinal chemistry 20071201 |
| Carbonic anhydrase inhibitors: the beta-carbonic anhydrase from Helicobacter pylori is a new target for sulfonamide and sulfamate inhibitors. | Bioorganic & medicinal chemistry letters 20070701 |
| Carbonic anhydrase inhibitors. DNA cloning, characterization, and inhibition studies of the human secretory isoform VI, a new target for sulfonamide and sulfamate inhibitors. | Journal of medicinal chemistry 20070125 |
| Microsphere-based protease assays and screening application for lethal factor and factor Xa. | Cytometry. Part A : the journal of the International Society for Analytical Cytology 20060501 |
| Carbonic anhydrase inhibitors: cloning and sulfonamide inhibition studies of a carboxyterminal truncated alpha-carbonic anhydrase from Helicobacter pylori. | Bioorganic & medicinal chemistry letters 20060415 |
| QSAR study on topically acting sulfonamides incorporating GABA moieties: a molecular connectivity approach. | Bioorganic & medicinal chemistry letters 20060401 |
| Carbonic anhydrase inhibitors: DNA cloning and inhibition studies of the alpha-carbonic anhydrase from Helicobacter pylori, a new target for developing sulfonamide and sulfamate gastric drugs. | Journal of medicinal chemistry 20060323 |
| Carbonic anhydrase inhibitors. The mitochondrial isozyme VB as a new target for sulfonamide and sulfamate inhibitors. | Journal of medicinal chemistry 20051201 |
| Carbonic anhydrase inhibitors: inhibition of the transmembrane isozyme XIV with sulfonamides. | Bioorganic & medicinal chemistry letters 20050901 |
| Carbonic anhydrase inhibitors. Inhibition of the transmembrane isozyme XII with sulfonamides-a new target for the design of antitumor and antiglaucoma drugs? | Bioorganic & medicinal chemistry letters 20050215 |
| Carbonic anhydrase inhibitors. Inhibition of the human cytosolic isozyme VII with aromatic and heterocyclic sulfonamides. | Bioorganic & medicinal chemistry letters 20050215 |
| Carbonic anhydrase inhibitors: inhibition of human cytosolic isozyme II and mitochondrial isozyme V with a series of benzene sulfonamide derivatives. | Bioorganic & medicinal chemistry letters 20041115 |
| Carbonic anhydrase inhibitors. Inhibition of cytosolic isozyme XIII with aromatic and heterocyclic sulfonamides: a novel target for the drug design. | Bioorganic & medicinal chemistry letters 20040716 |
| Carbonic anhydrase inhibitors: the first QSAR study on inhibition of tumor-associated isoenzyme IX with aromatic and heterocyclic sulfonamides. | Bioorganic & medicinal chemistry letters 20040621 |
| Carbonic anhydrase inhibitors: inhibition of the tumor-associated isozyme IX with aromatic and heterocyclic sulfonamides. | Bioorganic & medicinal chemistry letters 20030324 |
| Carbonic anhydrase inhibitors. A general approach for the preparation of water-soluble sulfonamides incorporating polyamino-polycarboxylate tails and of their metal complexes possessing long-lasting, topical intraocular pressure-lowering properties. | Journal of medicinal chemistry 20020328 |
| Carbonic anhydrase inhibitors: synthesis of sulfonamides incorporating dtpa tails and of their zinc complexes with powerful topical antiglaucoma properties. | Bioorganic & medicinal chemistry letters 20010226 |