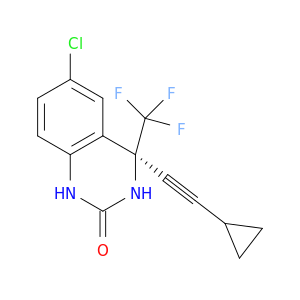
(4S)-6-Chloro-4-(2-cyclopropylethynyl)-3,4-dihydro-4-(trifluoromethyl)-2(1H)-quinazolinone
| Title | Journal |
|---|---|
| A novel series of (S)-2,7-substituted-1,2,3,4-tetrahydroisoquinoline-3-carboxylic acids: peroxisome proliferator-activated receptor α/γ dual agonists with protein-tyrosine phosphatase 1B inhibitory activity. | Chemical & pharmaceutical bulletin 20110101 |
| QSAR for non-nucleoside inhibitors of HIV-1 reverse transcriptase. | Bioorganic & medicinal chemistry 20060901 |
| General scope of 1,4-diastereoselective additions to a 2(3H)-quinazolinone: practical preparation of HIV therapeutics. | The Journal of organic chemistry 20030207 |
| Potency of nonnucleoside reverse transcriptase inhibitors (NNRTIs) used in combination with other human immunodeficiency virus NNRTIs, NRTIs, or protease inhibitors. | Antimicrobial agents and chemotherapy 20020601 |
| 4,1-Benzoxazepinone analogues of efavirenz (Sustiva) as HIV-1 reverse transcriptase inhibitors. | Bioorganic & medicinal chemistry letters 20010604 |
| Trifluoromethyl-containing 3-alkoxymethyl- and 3-aryloxymethyl-2-pyridinones are potent inhibitors of HIV-1 non-nucleoside reverse transcriptase. | Bioorganic & medicinal chemistry letters 20010212 |
| 3,3a-Dihydropyrano[4,3,2-de]quinazolin-2(1H)-ones are potent non-nucleoside reverse transcriptase inhibitors. | Bioorganic & medicinal chemistry letters 20010122 |
| Inhibition of clinically relevant mutant variants of HIV-1 by quinazolinone non-nucleoside reverse transcriptase inhibitors. | Journal of medicinal chemistry 20000518 |
| Novel 2,2-dioxide-4,4-disubstituted-1,3-H-2,1,3-benzothiadiazines as non-nucleoside reverse transcriptase inhibitors. | Bioorganic & medicinal chemistry letters 20000117 |
| Expanded-spectrum nonnucleoside reverse transcriptase inhibitors inhibit clinically relevant mutant variants of human immunodeficiency virus type 1. | Antimicrobial agents and chemotherapy 19991201 |