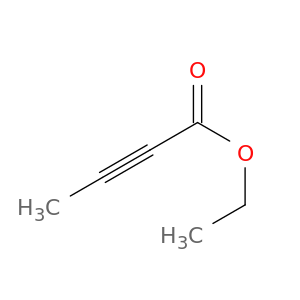
Ethyl 2-butynoate
| Title | Journal |
|---|---|
| Calyculins and related marine natural products as serine-threonine protein phosphatase PP1 and PP2A inhibitors and total syntheses of calyculin A, B, and C. | Marine drugs 20100101 |
| Isolation and crystal structures of both enol and keto tautomer intermediates in a hydration of an alkyne-carboxylic acid ester catalyzed by iridium complexes in water. | Journal of the American Chemical Society 20081217 |
| Increases in microbial nitrogen production and efficiency in vitro with three inhibitors of ruminal methanogenesis. | Canadian journal of microbiology 20070401 |
| Effects of butyrate precursors on electron relocation when methanogenesis is inhibited in ruminal mixed cultures. | Letters in applied microbiology 20060601 |
| Synthesis of carbocyclic hydantocidins via regioselective and diastereoselective phosphine-catalyzed [3 + 2]-cycloadditions to 5-methylenehydantoins. | The Journal of organic chemistry 20050805 |
| Effects of several inhibitors on pure cultures of ruminal methanogens. | Journal of applied microbiology 20040101 |
| Use of some novel alternative electron sinks to inhibit ruminal methanogenesis. | Reproduction, nutrition, development 20030101 |