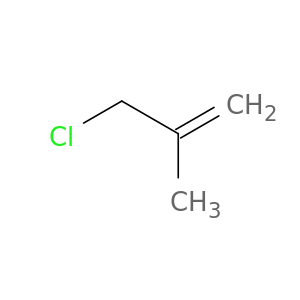
3-Chloro-2-methylpropene
| Title | Journal |
|---|---|
| Imidazolopiperazines: lead optimization of the second-generation antimalarial agents. | Journal of medicinal chemistry 20120510 |
| 3-Chloro-2-methylpropene. | Report on carcinogens : carcinogen profiles 20110101 |
| 8a-Methyl-5,6,8,8a,9,10-hexa-hydro-10,12a-epoxy-isoindolo[1,2-a]isoquinolinium iodide. | Acta crystallographica. Section E, Structure reports online 20100601 |
| Synthesis and effect on human HepG2 cells of 1,2-bis-(2-methylallyl)disulfane. | Molecules (Basel, Switzerland) 20100518 |
| Application of the margin of exposure (MoE) approach to substances in food that are genotoxic and carcinogenic - example: 1-methylcyclopropene and its impurities (1-chloro-2-methylpropene and 3-chloro-2-methylpropene). | Food and chemical toxicology : an international journal published for the British Industrial Biological Research Association 20100101 |
| Redesigning dehalogenase access tunnels as a strategy for degrading an anthropogenic substrate. | Nature chemical biology 20091001 |
| Quantitative structure-activity relationships for toxicity and genotoxicity of halogenated aliphatic compounds: wing spot test of Drosophila melanogaster. | Chemosphere 20070201 |
| Dechlorination of beta-methylallyl chloride by electrogenerated [Co(I)(bipyridine)3]+: an electrochemical study in the presence of cationic surfactants. | Journal of colloid and interface science 20060515 |
| Experimental and theoretical studies on formation and degradation of chloro organic compounds. | Chemosphere 20060301 |
| Cyclodextrins containing an acetone bridge. Synthesis and study as epoxidation catalysts. | Organic & biomolecular chemistry 20041207 |
| Highly regio- and chemoselective palladium-catalyzed propargylallylation of activated olefins: a novel route to 1,7-enyne derivatives. | The Journal of organic chemistry 20040611 |
| 3-Chloro-2-methylpropene. | Report on carcinogens : carcinogen profiles 20040101 |
| 3-Chloro-2-methylpropene. | Report on carcinogens : carcinogen profiles 20020101 |
| Point mutations of K-ras and H-ras genes in forestomach neoplasms from control B6C3F1 mice and following exposure to 1,3-butadiene, isoprene or chloroprene for up to 2-years. | Chemico-biological interactions 20010601 |
| Chronic inhalation toxicity and carcinogenicity studies of 3-chloro-2-methylpropene in BDF1 mice. | Industrial health 20000701 |
| Comparative metabolism and disposition of 1-chloro- and 3-chloro-2-methylpropene in rats and mice. | Drug metabolism and disposition: the biological fate of chemicals 19870101 |
| Forestomach lesions in rats and mice administered 3-chloro-2-methylpropene by gavage for two years. | Cancer research 19861201 |
| Association of chemically induced forestomach cell proliferation and carcinogenesis. | Cancer letters 19860901 |