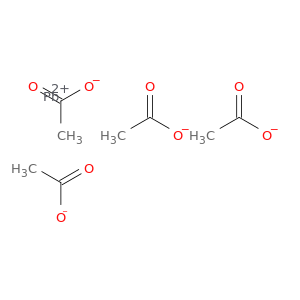
Lead tetraacetate; 5-10% of acetic acid
| Title | Journal |
|---|---|
| A Drosophila model for toxicogenomics: Genetic variation in susceptibility to heavy metal exposure. | PLoS genetics 20170701 |
| Coordinated waves of gene expression during neuronal differentiation of embryonic stem cells as basis for novel approaches to developmental neurotoxicity testing. | Cell death and differentiation 20110301 |
| Scope and mechanism of intramolecular aziridination of cyclopent-3-enyl-methylamines to 1-azatricyclo[2.2.1.0(2,6)]heptanes with lead tetraacetate. | Journal of the American Chemical Society 20090826 |
| First chemical synthesis of antioxidative metabolites of sesamin. | Chemical & pharmaceutical bulletin 20081101 |
| Cyclopentanes from N-amino-glyconolactams. A synthesis of mannostatin A. | Chemical communications (Cambridge, England) 20030421 |
| Formation of an unusual dimeric compound by lead tetraacetate oxidation of a corynanthe-type indole alkaloid, mitragynine. | Chemical & pharmaceutical bulletin 20020701 |
| Synthesis and characterization of nucleoside derivatives, n-(benzoyl)-n-(deoxyguanosin-8-yl)4-aminobiphenyl and n-(2'-deoxyguanosin-8-yl)4-aminobiphenyl via alpha-phenyl-n-(4-biphenyl)nitrone. | Nucleosides, nucleotides & nucleic acids 20020101 |