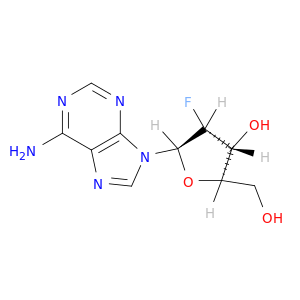
2'-Fluoro-2'-deoxyadenosine
| Title | Journal |
|---|---|
| S-Adenosylhomocysteine hydrolase of the protozoan parasite Trichomonas vaginalis: potent inhibitory activity of 9-(2-deoxy-2-fluoro-β,D-arabinofuranosyl)adenine. | Bioorganic & medicinal chemistry letters 20120615 |
| Susceptibility in vitro of clinically metronidazole-resistant Trichomonas vaginalis to nitazoxanide, toyocamycin, and 2-fluoro-2'-deoxyadenosine. | Parasitology research 20100901 |
| Structure of a mutant human purine nucleoside phosphorylase with the prodrug, 2-fluoro-2'-deoxyadenosine and the cytotoxic drug, 2-fluoroadenine. | Protein science : a publication of the Protein Society 20090501 |
| Unnatural substrates reveal the importance of 8-oxoguanine for in vivo mismatch repair by MutY. | Nature chemical biology 20080101 |
| Insight into the roles of tyrosine 82 and glycine 253 in the Escherichia coli adenine glycosylase MutY. | Biochemistry 20051101 |
| Structure-activity relationship of purine ribonucleosides for inhibition of hepatitis C virus RNA-dependent RNA polymerase. | Journal of medicinal chemistry 20040422 |
| Crystal structure and mechanism of a bacterial fluorinating enzyme. | Nature 20040205 |
| Insight into the functional consequences of inherited variants of the hMYH adenine glycosylase associated with colorectal cancer: complementation assays with hMYH variants and pre-steady-state kinetics of the corresponding mutated E.coli enzymes. | Journal of molecular biology 20030321 |
| Antitumor activity of 2-fluoro-2'-deoxyadenosine against tumors that express Escherichia coli purine nucleoside phosphorylase. | Cancer gene therapy 20030101 |
| A new synthesis of 9-(2-deoxy-2-fluoro-beta-D-arabinofuranosyl)guanine (AraF-G). | Nucleosides, nucleotides & nucleic acids 20030101 |
| Cell-free biosynthesis of fluoroacetate and 4-fluorothreonine in Streptomyces cattleya. | Angewandte Chemie (International ed. in English) 20021018 |
| CD80 antigen expression as a predictor of ex vivo chemosensitivity in chronic lymphocytic leukemia. | Leukemia research 20020501 |
| Biochemistry: biosynthesis of an organofluorine molecule. | Nature 20020321 |
| Synthesis and anti-hepatitis B virus activity of 9-(2-deoxy-2-fluoro-beta-L-arabinofuranosyl) purine nucleosides. | Journal of medicinal chemistry 19970815 |
| Purine 2'-deoxy-2'-fluororibosides as antiinfluenza virus agents. | Journal of medicinal chemistry 19930108 |