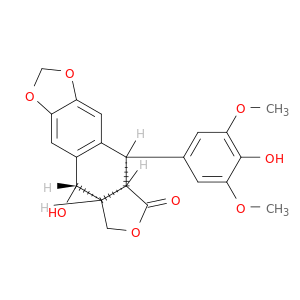
(10R,11R,15R,16S)-16-hydroxy-10-(4-hydroxy-3,5-dimethoxyphenyl)-4,6,13-trioxatetracyclo[7.7.0.0^{3,7}.0^{11,15}]hexadeca-1,3(7),8-trien-12-one
| Title | Journal |
|---|---|
| Six enzymes from mayapple that complete the biosynthetic pathway to the etoposide aglycone. | Science (New York, N.Y.) 20150911 |
| Exploration of natural compounds as sources of new bifunctional scaffolds targeting cholinesterases and beta amyloid aggregation: the case of chelerythrine. | Bioorganic & medicinal chemistry 20121115 |
| Manipulation of heterogeneity product in 4'-demethylepipodophyllotoxin biotransformation process by using yeast extract as nitrogen source. | Applied microbiology and biotechnology 20120101 |
| A novel biotransformation process of 4'-demethylepipodophyllotoxin to 4'-demethylepipodophyllic acid by Bacillus fusiformis CICC 20463, Part II: process optimization. | Bioprocess and biosystems engineering 20100201 |
| Synthesis and biological activity of novel shikonin analogues. | Bioorganic & medicinal chemistry letters 20090201 |
| Substituents on etoposide that interact with human topoisomerase IIalpha in the binary enzyme-drug complex: contributions to etoposide binding and activity. | Biochemistry 20080415 |
| A fragmentation study of two compounds related to 4'-demethylepipodophyllotoxin in negative ion electrospray ionization by MSn ion-trap time-of-flight mass spectrometry. | Rapid communications in mass spectrometry : RCM 20080101 |
| Antioxidative and antitumor activity of derivatives of 4-beta-amino-4'-demethylepipodophyllotoxin and their structure-activity relationship. | Die Pharmazie 20070601 |
| Synthesis and biological study of a new series of 4'-demethylepipodophyllotoxin derivatives. | Journal of medicinal chemistry 20050127 |
| Synthesis and cytotoxic activity of novel derivatives of 4'-demethylepipodophyllotoxin. | Bioorganic & medicinal chemistry letters 20041018 |
| Anti-AIDS agents. Part 61: Anti-HIV activity of new podophyllotoxin derivatives. | Bioorganic & medicinal chemistry 20040801 |
| Antitumor agents. Part 227: Studies on novel 4'-O-demethyl-epipodophyllotoxins as antitumor agents targeting topoisomerase II. | Bioorganic & medicinal chemistry 20040615 |
| Antitumor agents. 213. Modeling of epipodophyllotoxin derivatives using variable selection k nearest neighbor QSAR method. | Journal of medicinal chemistry 20020523 |