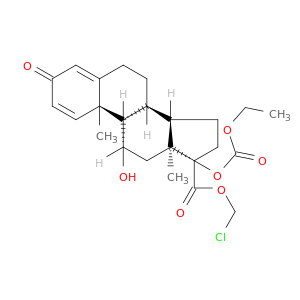
(11b,17a)-17-[(Ethoxycarbonyl)oxy]-11-hydroxy-3-oxo-Androsta-1,4-diene-17-carboxylic acid chloromethyl ester
| Title | Journal |
|---|---|
| Positional requirements for the stimulation of mRNA nuclear export by ALREX-promoting elements. | Molecular bioSystems 20121001 |
| Loteprednol etabonate ophthalmic suspension 0.5 %: efficacy and safety for postoperative anti-inflammatory use. | International ophthalmology 20121001 |
| A rabbit model of age-dependant ocular hypertensive response to topical corticosteroids. | Acta ophthalmologica 20120901 |
| Management of seasonal allergic conjunctivitis: guide to therapy. | Acta ophthalmologica 20120801 |
| Topical loteprednol etabonate 0.5 % for treatment of vernal keratoconjunctivitis: efficacy and safety. | Japanese journal of ophthalmology 20120701 |
| Loteprednol etabonate suspension 0.2% administered QID compared with olopatadine solution 0.1% administered BID in the treatment of seasonal allergic conjunctivitis: a multicenter, randomized, investigator-masked, parallel group study in Chinese patients. | Clinical therapeutics 20120601 |
| The effects of delta1-cortienic acid on skin blanching, pharmacokinetics and stability of loteprednol etabonate. | Die Pharmazie 20120501 |
| Safety and tolerability of loteprednol etabonate 0.5% and tobramycin 0.3% ophthalmic suspension in pediatric subjects. | Paediatric drugs 20120401 |
| A multicenter, randomized, parallel-group, clinical trial comparing the safety and efficacy of loteprednol etabonate 0.5%/tobramycin 0.3% with dexamethasone 0.1%/tobramycin 0.3% in the treatment of Chinese patients with blepharokeratoconjunctivitis. | Current medical research and opinion 20120301 |
| [Study on the treatment of dry eye with Loteprednol Etabonate]. | [Zhonghua yan ke za zhi] Chinese journal of ophthalmology 20120201 |
| Advances in corticosteroid therapy for ocular inflammation: loteprednol etabonate. | International journal of inflammation 20120101 |
| Long-term results of oral valganciclovir for treatment of anterior segment inflammation secondary to cytomegalovirus infection. | Clinical ophthalmology (Auckland, N.Z.) 20120101 |
| Use of loteprednol for routine prophylaxis after photorefractive keratectomy. | Clinical ophthalmology (Auckland, N.Z.) 20120101 |
| Loteprednol etabonate gel 0.5% for postoperative pain and inflammation after cataract surgery: results of a multicenter trial. | Clinical ophthalmology (Auckland, N.Z.) 20120101 |
| Prediction of drug combinations by integrating molecular and pharmacological data. | PLoS computational biology 20111201 |
| Microsporidial stromal keratitis successfully treated with medical therapy: a case report. | Cornea 20111101 |
| Steroid-induced ocular hypertension with loteprednol etabonate 0.2%--a case report. | Optometry (St. Louis, Mo.) 20110701 |
| Dry eye syndrome. | Journal of ophthalmic & vision research 20110701 |
| Intraocular pressure elevations with loteprednol etabonate: a retrospective chart review. | Journal of ocular pharmacology and therapeutics : the official journal of the Association for Ocular Pharmacology and Therapeutics 20110601 |
| A convenient synthesis of the side chain of loteprednol etabonate--an ocular soft corticosteroid from 20-oxopregnanes using metal-mediated halogenation as a key reaction. | Steroids 20110401 |
| Genome analysis reveals interplay between 5'UTR introns and nuclear mRNA export for secretory and mitochondrial genes. | PLoS genetics 20110401 |
| Topical loteprednol pretreatment reduces cyclosporine stinging in chronic dry eye disease. | Journal of ocular pharmacology and therapeutics : the official journal of the Association for Ocular Pharmacology and Therapeutics 20110201 |
| Safety and efficacy of loteprednol etabonate ophthalmic ointment 0.5% for the treatment of inflammation and pain following cataract surgery. | Clinical ophthalmology (Auckland, N.Z.) 20110101 |
| Treating ocular surface disease: new agents in development. | Clinical ophthalmology (Auckland, N.Z.) 20110101 |
| A local glucose-and oxygen concentration-based insulin secretion model for pancreatic islets. | Theoretical biology & medical modelling 20110101 |
| Critical appraisal of ophthalmic ketorolac in treatment of pain and inflammation following cataract surgery. | Clinical ophthalmology (Auckland, N.Z.) 20110101 |
| Current status of immunomodulatory and cellular therapies in preclinical and clinical islet transplantation. | Journal of transplantation 20110101 |
| Combination therapies in ophthalmology: implications for intravitreal delivery. | Journal of ophthalmic & vision research 20110101 |
| Intraocular pressure elevation from topical difluprednate use. | Optometry (St. Louis, Mo.) 20101201 |
| Treatment of seasonal allergic conjunctivitis with ophthalmic corticosteroids: in search of the perfect ocular corticosteroids in the treatment of allergic conjunctivitis. | Current opinion in allergy and clinical immunology 20101001 |
| Exacerbation of zoster interstitial keratitis after zoster vaccination in an adult. | Archives of ophthalmology (Chicago, Ill. : 1960) 20100801 |
| Pharmacokinetics and delta1-cortienic acid excretion after intravenous administration of prednisolone and loteprednol etabonate in rats. | Die Pharmazie 20100601 |
| Feasibility of localized immunosuppression: 1. Exploratory studies with glucocorticoids in a biohybrid device designed for cell transplantation. | Die Pharmazie 20100601 |
| Feasibility of localized immunosuppression: 2. PLA microspheres for the sustained local delivery of a soft immunosuppressant. | Die Pharmazie 20100601 |
| Loteprednol and tobramycin in combination: a review of their impact on current treatment regimens. | Expert opinion on pharmacotherapy 20100401 |
| Use of rituximab for periocular and intraocular mucosa-associated lymphoid tissue lymphoma. | Ocular immunology and inflammation 20100401 |
| Concentrations of besifloxacin, gatifloxacin, and moxifloxacin in human conjunctiva after topical ocular administration. | Clinical ophthalmology (Auckland, N.Z.) 20100101 |
| Attenuation of ocular hypertension with the use of topical loteprednol etabonate 0.5% in steroid responders after corneal transplantation. | Cornea 20091201 |
| Conjunctival lichen planus in a patient with herpes simplex virus keratitis. | Cornea 20090901 |
| Steroid-induced intraocular pressure elevation or glaucoma after penetrating keratoplasty in patients with keratoconus or Fuchs dystrophy. | Cornea 20090801 |
| FEM-based oxygen consumption and cell viability models for avascular pancreatic islets. | Theoretical biology & medical modelling 20090101 |
| BOL-303242-X, a novel selective glucocorticoid receptor agonist, with full anti-inflammatory properties in human ocular cells. | Molecular vision 20090101 |
| Etiology and treatment of the inflammatory causes of cystoid macular edema. | Journal of inflammation research 20090101 |
| Treatment of chronic dry eye: focus on cyclosporine. | Clinical ophthalmology (Auckland, N.Z.) 20081201 |
| Treatment of multidrug-resistant Flavobacterium indologenes keratitis with trimethoprim-sulfamethoxazole. | Cornea 20081001 |
| Tolerability of loteprednol/tobramycin versus dexamethasone/tobramycin in healthy volunteers: results of a 4-week, randomized, double-masked, parallel-group study. | Current medical research and opinion 20080801 |
| Potential bias in ophthalmic pharmaceutical clinical trials. | Clinical ophthalmology (Auckland, N.Z.) 20080601 |
| Treatment of ocular inflammatory conditions with loteprednol etabonate. | The British journal of ophthalmology 20080401 |
| Pharmacokinetics of the sequential metabolites of loteprednol etabonate in rats. | The Journal of pharmacy and pharmacology 20080301 |
| Soft corticosteroids for local immunosuppression: exploring the possibility for the use of loteprednol etabonate for islet transplantation. | Die Pharmazie 20080301 |
| Comparison of the safety and efficacy of loteprednol 0.5%/tobramycin 0.3% with dexamethasone 0.1%/tobramycin 0.3% in the treatment of blepharokeratoconjunctivitis. | Current medical research and opinion 20080101 |
| Effects of loteprednol/tobramycin versus dexamethasone/tobramycin on intraocular pressure in healthy volunteers. | Cornea 20080101 |
| Periocular necrotizing fasciitis associated with kerato-conjunctivitis and treated with medical management: a case report. | Indian journal of ophthalmology 20080101 |
| A review of the use of ketorolac tromethamine 0.4% in the treatment of post-surgical inflammation following cataract and refractive surgery. | Clinical ophthalmology (Auckland, N.Z.) 20071201 |
| Comparison of tobramycin 0.3%/dexamethasone 0.1% and tobramycin 0.3%/loteprednol 0.5% in the management of blepharo-keratoconjunctivitis. | Advances in therapy 20070101 |
| Pseudodendritic keratitis in ocular rosacea causing a diagnostic dilemma. | Indian journal of ophthalmology 20070101 |
| Conjunctival non-caseating granulomas in a human immunodeficiency virus (HIV) positive patient attributed to sarcoidosis. | Ocular immunology and inflammation 20061001 |
| Comparison of ketorolac tromethamine, diclofenac sodium, and loteprednol etabonate in an animal model of ocular inflammation. | Journal of ocular pharmacology and therapeutics : the official journal of the Association for Ocular Pharmacology and Therapeutics 20060601 |
| Prevention and treatment of corneal graft rejection: current practice patterns (2004). | Cornea 20060401 |
| Corticosteroid design for the treatment of asthma: structural insights and the therapeutic potential of soft corticosteroids. | Current pharmaceutical design 20060101 |
| Ophthalmic drug design based on the metabolic activity of the eye: soft drugs and chemical delivery systems. | The AAPS journal 20051201 |
| Dendritic keratopathy in ocular rosacea. | Cornea 20050701 |
| Effect of loteprednol etabonate nasal spray suspension on seasonal allergic rhinitis assessed by allergen challenge in an environmental exposure unit. | Allergy 20050301 |
| Comparison of topical steroids for acute anterior uveitis. | Journal of ocular pharmacology and therapeutics : the official journal of the Association for Ocular Pharmacology and Therapeutics 20041201 |
| An HPLC method to evaluate purity of a steroidal drug, loteprednol etabonate. | Journal of pharmaceutical and biomedical analysis 20041029 |
| Developments in ocular allergy. | Current opinion in allergy and clinical immunology 20041001 |
| A randomized, double-masked, placebo-controlled, multicenter comparison of loteprednol etabonate ophthalmic suspension, 0.5%, and placebo for treatment of keratoconjunctivitis sicca in patients with delayed tear clearance. | American journal of ophthalmology 20040901 |
| Intranasal loteprednol etabonate in healthy male subjects: pharmacokinetics and effects on endogenous cortisol. | Journal of clinical pharmacology 20040501 |
| Possibilities in improvement of glucocorticoid treatments in asthma with special reference to loteprednol etabonate. | Die Pharmazie 20040501 |
| Isolation and structure elucidation of the major photodegradation products of loteprednol etabonate. | Steroids 20040101 |
| Long-term safety of loteprednol etabonate 0.2% in the treatment of seasonal and perennial allergic conjunctivitis. | Eye & contact lens 20040101 |
| Acute anterior uveitis. | Clinical evidence 20031201 |
| Peripheral hypertrophic subepithelial corneal degeneration. | Eye & contact lens 20031001 |
| Readability of ocular medication inserts. | Journal of glaucoma 20030201 |
| Corneal juvenile xanthogranuloma in a 4-month-old child. | Archives of ophthalmology (Chicago, Ill. : 1960) 20030101 |
| Soft steroids: a new approach to the treatment of inflammatory airways diseases. | Pulmonary pharmacology & therapeutics 20030101 |
| Compared topical ocular olopatadine 0.1% (Patanol) and loteprednol etabonate 0.2% (Alrex) in an allergen challenge model. | Clinical therapeutics 20020901 |
| Comparison of the clinical efficacy and tolerability of olopatadine hydrochloride 0.1% ophthalmic solution and loteprednol etabonate 0.2% ophthalmic suspension in the conjunctival allergen challenge model. | Clinical therapeutics 20020601 |
| Comparison of ketorolac tromethamine 0.5% and loteprednol etabonate 0.5% for inflammation after phacoemulsification: prospective randomized double-masked study. | Journal of cataract and refractive surgery 20020101 |
| Placement of a collagen glaucoma drainage device to control intraocular pressure and chronic iritis secondary to juvenile rheumatoid arthritis. | Ophthalmic surgery and lasers 20020101 |
| Loteprednol etabonate. Alrex, Lotemax, Loteprednol, Loterox. | Drugs in R&D 20020101 |
| Retrometabolic drug design--novel aspects, future directions. | Die Pharmazie 20011001 |
| Pharmacological validation of a feline model of steroid-induced ocular hypertension. | Archives of ophthalmology (Chicago, Ill. : 1960) 19990301 |