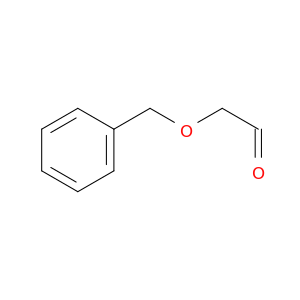
Benzyloxyacetaldehyde
| Title | Journal |
|---|---|
| Catalysis through temporary intramolecularity: mechanistic investigations on aldehyde-catalyzed Cope-type hydroamination lead to the discovery of a more efficient tethering catalyst. | Journal of the American Chemical Society 20121010 |
| Generating a generation of proteasome inhibitors: from microbial fermentation to total synthesis of salinosporamide a (marizomib) and other salinosporamides. | Marine drugs 20100101 |
| Synthetic studies on (-)-lemonomycin: an efficient asymmetric synthesis of lemonomycinone amide. | The Journal of organic chemistry 20090306 |
| D-fructose-6-phosphate aldolase in organic synthesis: cascade chemical-enzymatic preparation of sugar-related polyhydroxylated compounds. | Chemistry (Weinheim an der Bergstrasse, Germany) 20090101 |
| Serine hydroxymethyl transferase from Streptococcus thermophilus and L-threonine aldolase from Escherichia coli as stereocomplementary biocatalysts for the synthesis of beta-hydroxy-alpha,omega-diamino acid derivatives. | Chemistry (Weinheim an der Bergstrasse, Germany) 20080101 |
| Single diastereomers of polyhydroxylated 9-oxa-1-azabicyclo[4.2.1]nonanes from intramolecular 1,3-dipolar cycloaddition of omega-unsaturated nitrones. | The Journal of organic chemistry 20061027 |
| Recombinant production of serine hydroxymethyl transferase from Streptococcus thermophilus and its preliminary evaluation as a biocatalyst. | Applied microbiology and biotechnology 20050901 |
| (R)-Goniothalamin: total syntheses and cytotoxic activity against cancer cell lines. | Bioorganic & medicinal chemistry 20050415 |
| Crystal structure of vinorine synthase, the first representative of the BAHD superfamily. | The Journal of biological chemistry 20050408 |
| Aldol additions of dihydroxyacetone phosphate to N-Cbz-amino aldehydes catalyzed by L-fuculose-1-phosphate aldolase in emulsion systems: inversion of stereoselectivity as a function of the acceptor aldehyde. | Chemistry (Weinheim an der Bergstrasse, Germany) 20050218 |
| Chiral 2,6-bis(oxazolinyl)pyridine-rare earth metal complexes as catalysts for highly enantioselective 1,3-dipolar cycloaddition reactions of 2-benzopyrylium-4-olates. | The Journal of organic chemistry 20050107 |
| An intramolecular 1,3-dipolar cycloaddition reaction towards the synthesis of chiral azetidine nucleoside analogues: the D-gluco case. | Nucleosides, nucleotides & nucleic acids 20050101 |
| Stereo- and regioselectivity of Pd(0)/InI-mediated allylic additions to aldehydes and ketones. In situ generation of allylindium(III) intermediates from N-acylnitroso Diels-Alder cycloadducts and 1-amino-4-acetoxycyclopentenes. | The Journal of organic chemistry 20030110 |
| Highly enantioselective 1,3-dipolar cycloaddition reactions of 2-benzopyrylium-4-olate catalyzed by chiral Lewis acids. | Journal of the American Chemical Society 20021218 |