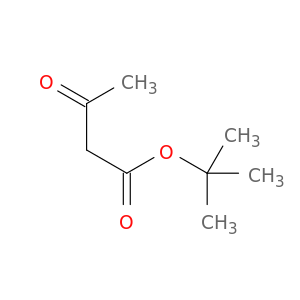
tert-Butyl acetoacetate
| Title | Journal |
|---|---|
| Synthesis of indenoporphyrins, highly modified porphyrins with reduced diatropic characteristics. | The Journal of organic chemistry 20110701 |
| Improved synthesis of three methyl-branched pheromone components produced by the female lichen moth. | Bioscience, biotechnology, and biochemistry 20100101 |
| Synthesis of cyclopentenones via intramolecular HWE and the palladium-catalyzed reactions of allylic hydroxy phosphonate derivatives. | The Journal of organic chemistry 20080718 |
| An alternative to the use of delta-lactam urethanes in the 'ring switch' approach to higher homologues of AMPA-type glutamate antagonists. | Organic & biomolecular chemistry 20030807 |