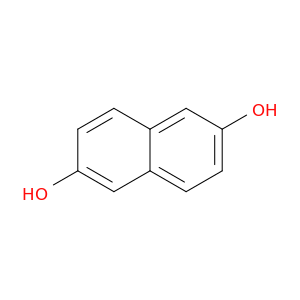
2,6-Dihydroxynaphthalene
| Title | Journal |
|---|---|
| A light-controlled molecular brake with complete ON-OFF rotation. | Chemistry (Weinheim an der Bergstrasse, Germany) 20100315 |
| Synthesis, properties, crystal structures, and semiconductor characteristics of naphtho[1,2-b:5,6-b']dithiophene and -diselenophene derivatives. | The Journal of organic chemistry 20100219 |
| New thermal and microbial resistant metal-containing epoxy polymers. | Bioinorganic chemistry and applications 20100101 |
| Chromane derivatives of small aromatic molecules: Chemoenzymatic synthesis and growth inhibitory activity on human tumor cell line LoVo WT. | Bioorganic & medicinal chemistry 20090815 |
| Experimental and theoretical studies on constitutional isomers of 2,6-dihydroxynaphthalene carbaldehydes. Effects of resonance-assisted hydrogen bonding on the electronic absorption spectra. | The Journal of organic chemistry 20090116 |
| 4,4'-(2,6-Dihydroxy-naphthalene-1,5-diyldimethyl-ene)dipyridinium bis-(per-chlorate). | Acta crystallographica. Section E, Structure reports online 20080601 |
| Toward high-generation rotaxane dendrimers that incorporate a ring component on every branch: noncovalent synthesis of a dendritic [10]pseudorotaxane with 13 molecular components. | Chemistry, an Asian journal 20070604 |
| Selective antiproliferative activity of hydroxynaphthyl-beta-D-xylosides. | Journal of medicinal chemistry 20060323 |
| The influence of aromatic residues in hydraphile spacer units: assay by ion selective electrode methods and in bacteria. | Bioorganic & medicinal chemistry 20050502 |
| Hydroxynaphthaldehyde phosphate derivatives as potent covalent Schiff base inhibitors of fructose-1,6-bisphosphate aldolase. | Biochemistry 20050412 |
| Regiospecific oxidation of naphthalene and fluorene by toluene monooxygenases and engineered toluene 4-monooxygenases of Pseudomonas mendocina KR1. | Biotechnology and bioengineering 20050405 |
| Oxidase enzyme immobilisation through electropolymerised films to assemble biosensors for batch and flow injection analysis. | Biosensors & bioelectronics 20030501 |
| Novel solid-state polycondensation I. Oxidative-coupling polymerization of 2,6-dihydroxynaphthalene. | Chemical communications (Cambridge, England) 20020121 |
| A covalent vanadium(III) 2-dimensional network and vanadyl chains linked by aryldioxides. | Inorganic chemistry 20010115 |