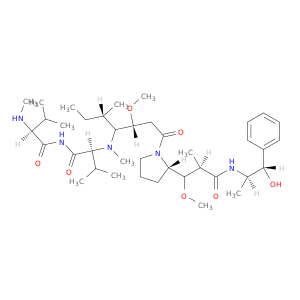
Monomethyl auristatin e
| Title | Journal |
|---|---|
| Native intact mass determination of antibodies conjugated with monomethyl Auristatin E and F at interchain cysteine residues. | Analytical chemistry 20120320 |
| Targeting cell surface alpha(v)beta(3) integrin increases therapeutic efficacies of a legumain protease-activated auristatin prodrug. | Molecular pharmaceutics 20120101 |
| Brentuximab vedotin and crizotinib in anaplastic large-cell lymphoma. | Cancer journal (Sudbury, Mass.) 20120101 |
| Glembatumumab vedotin, a conjugate of an anti-glycoprotein non-metastatic melanoma protein B mAb and monomethyl auristatin E for the treatment of melanoma and breast cancer. | Current opinion in molecular therapeutics 20100401 |
| EphA2 immunoconjugate as molecularly targeted chemotherapy for ovarian carcinoma. | Journal of the National Cancer Institute 20090902 |
| Potent antitumor activity of the anti-CD19 auristatin antibody drug conjugate hBU12-vcMMAE against rituximab-sensitive and -resistant lymphomas. | Blood 20090430 |
| Site-specific conjugation of a cytotoxic drug to an antibody improves the therapeutic index. | Nature biotechnology 20080801 |
| Combination of the anti-CD30-auristatin-E antibody-drug conjugate (SGN-35) with chemotherapy improves antitumour activity in Hodgkin lymphoma. | British journal of haematology 20080701 |
| Engineered antibody-drug conjugates with defined sites and stoichiometries of drug attachment. | Protein engineering, design & selection : PEDS 20060701 |
| Potent cytotoxicity of an auristatin-containing antibody-drug conjugate targeting melanoma cells expressing melanotransferrin/p97. | Molecular cancer therapeutics 20060601 |
| Lymphocyte activation antigen CD70 expressed by renal cell carcinoma is a potential therapeutic target for anti-CD70 antibody-drug conjugates. | Cancer research 20060215 |
| Evaluation of RGD-targeted albumin carriers for specific delivery of auristatin E to tumor blood vessels. | Bioconjugate chemistry 20060101 |
| Efficient elimination of B-lineage lymphomas by anti-CD20-auristatin conjugates. | Clinical cancer research : an official journal of the American Association for Cancer Research 20041201 |