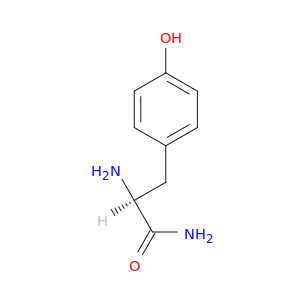
(2S)-2-amino-3-(4-hydroxyphenyl)propanamide
| Title | Journal |
|---|---|
| Simple and highly enantioselective electrochemical aptamer-based binding assay for trace detection of chiral compounds. | Analytical chemistry 20120619 |
| Design of novel tyrosine-nitrogen mustard hybrid molecules active against uterine, ovarian and breast cancer cell lines. | Steroids 20120401 |
| Fluorescence polarization biosensor based on an aptamer enzymatic cleavage protection strategy. | Analytical and bioanalytical chemistry 20111201 |
| Optimization of the structure-switching aptamer-based fluorescence polarization assay for the sensitive tyrosinamide sensing. | Analytica chimica acta 20111130 |
| Aptamer enzymatic cleavage protection assay for the gold nanoparticle-based colorimetric sensing of small molecules. | Analytica chimica acta 20111114 |
| Glycan labeling strategies and their use in identification and quantification. | Analytical and bioanalytical chemistry 20100801 |
| Inhibition of Ser/Thr phosphatases induces capacitation-associated signaling in the presence of Src kinase inhibitors. | The Journal of biological chemistry 20100312 |
| Design strategies of fluorescent biosensors based on biological macromolecular receptors. | Sensors (Basel, Switzerland) 20100101 |
| Noncompetitive fluorescence polarization aptamer-based assay for small molecule detection. | Analytical chemistry 20090901 |
| Simultaneous delivery of doxorubicin and gemcitabine to tumors in vivo using prototypic polymeric drug carriers. | Biomaterials 20090701 |
| Identification of tyrosine 806 as a molecular determinant of RET kinase sensitivity to ZD6474. | Endocrine-related cancer 20090301 |
| Synthesis, spectroscopic and structural elucidation of tyrosinamide hydrogensquarate monohydrate. | Amino acids 20090201 |
| Electropolymerized tyrosine-based thin films: selective cell binding via peptide recognition to novel electropolymerized biomimetic tyrosine RGDY films. | Analytical biochemistry 20090101 |
| Image-guided and passively tumour-targeted polymeric nanomedicines for radiochemotherapy. | British journal of cancer 20080916 |
| Microcalorimetrics studies of the thermodynamics and binding mechanism between L-tyrosinamide and aptamer. | The journal of physical chemistry. B 20080529 |
| Physiological variations of stem cell factor and stromal-derived factor-1 in murine models of liver injury and regeneration. | Liver international : official journal of the International Association for the Study of the Liver 20080301 |
| CE with electrochemical detection for investigation of label-free recognition of amino acid amides by guanine-rich DNA aptamers. | Electrophoresis 20070801 |
| Antineoplastic agents. 515. Synthesis of human cancer cell growth inhibitors derived from 3,4-methylenedioxy-5,4'-dimethoxy-3'-amino-Z-stilbene. | Journal of natural products 20050801 |
| NF-kappaB pathway protects cochlear hair cells from aminoglycoside-induced ototoxicity. | Journal of neuroscience research 20050301 |
| Influence of alkyl group on amide nitrogen atom on fluorescence quenching of tyrosine amide and N-acetyltyrosine amide. | Biophysical chemistry 20041001 |
| Immobilized DNA aptamers as target-specific chiral stationary phases for resolution of nucleoside and amino acid derivative enantiomers. | Analytical chemistry 20040215 |
| Enzymatic copolymerization alters the structure of unpolymerized mixtures of the biomimetic monomers: the amphiphilic decyl ester of L-tyrosine and L-tyrosineamide--an AFM investigation of nano- to micrometer-scale structure differences. | Biomacromolecules 20040101 |
| Chemolabile cellular microarrays for screening small molecules and peptides. | Molecular diversity 20040101 |
| Antineoplastic agents. 487. Synthesis and biological evaluation of the antineoplastic agent 3,4-methylenedioxy-5,4'-dimethoxy-3'-amino-Z-stilbene and derived amino acid amides. | Journal of medicinal chemistry 20030213 |
| Probing the binding specificity of C-type lectins in vivo. | Methods in enzymology 20030101 |
| Spectroscopic properties of tyrosyl radicals in dipeptides. | Journal of the American Chemical Society 20020515 |
| In vitro selection of DNA aptamers that bind L-tyrosinamide. | Bioorganic & medicinal chemistry 20011001 |