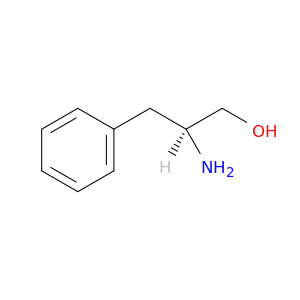
L-Phenylalaninol
| Title | Journal |
|---|---|
| 20-Residue and 11-residue peptaibols from the fungus Trichoderma longibrachiatum are synergistic in forming Na+/K+ -permeable channels and adverse action towards mammalian cells. | The FEBS journal 20121101 |
| A colorimetric chiral sensor based on chiral crown ether for the recognition of the two enantiomers of primary amino alcohols and amines. | Chirality 20110401 |
| A Standard Protocol for the Calibration of Capillary Electrophoresis (CE) Equipment. | Scientia pharmaceutica 20110101 |
| Absolute configurations and CD spectra of axially chiral biphenyls prepared in a facile manner by crystallization-induced configuration transformation. | Chirality 20100801 |
| Synthesis of enantiopure 2-aryl-2-methoxypropionic acids and determination of their absolute configurations by X-ray crystallography. | Chirality 20080301 |
| Direct high-performance liquid chromatographic separation of the enantiomers of an aromatic amine and four aminoalcohols using polysaccharide chiral stationary phases and acidic additive. | Chirality 20070801 |
| Straightforward methodology for the enantioselective synthesis of benzo[a]- and indolo[2,3-a]quinolizidines. | The Journal of organic chemistry 20070706 |
| New route to enantiopure MalphaNP acid, a powerful resolution and chiral 1H NMR anisotropy reagent. | Chirality 20070515 |
| Microsphere-based protease assays and screening application for lethal factor and factor Xa. | Cytometry. Part A : the journal of the International Society for Analytical Cytology 20060501 |
| Synthesis and activity of 2-oxoamides containing long chain beta-amino acids. | Journal of peptide science : an official publication of the European Peptide Society 20050701 |
| Chiral oxime ethers in asymmetric synthesis. O-(1-Phenylbutyl)benzyloxyacetaldoxime, a versatile reagent for the asymmetric synthesis of protected 1,2-aminoalcohols, alpha-amino acid derivatives, and 2-hydroxymethyl nitrogen heterocycles including iminosugars. | Organic & biomolecular chemistry 20050407 |
| Chiral bis(amino alcohol)oxalamide gelators-gelation properties and supramolecular organization: racemate versus pure enantiomer gelation. | Chemistry (Weinheim an der Bergstrasse, Germany) 20031121 |
| Identification of a novel system L amino acid transporter structurally distinct from heterodimeric amino acid transporters. | The Journal of biological chemistry 20031031 |
| Resolution of 1- and 2-naphthylmethoxyacetic acids, NMR reagents for absolute configuration determination, by use of L-phenylalaninol. | Chirality 20030801 |
| Tuning and enhancing enantioselective quenching of calixarene hosts by chiral guest amines. | Analytical chemistry 20020101 |
| Enantioselective molecular sensing of aromatic amines using tetra-(S)-di-2-naphthylprolinol calix[4]arene. | The Analyst 20010701 |
| Chemotactic peptide analogues. Synthesis and chemotactic activity of N-formyl-Met-Leu-Phe analogues containing (S)-phenylalaninol derivatives. | Archiv der Pharmazie 19950901 |