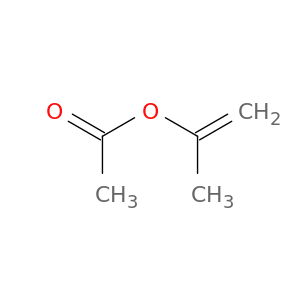
Prop-1-en-2-yl acetate
| Title | Journal |
|---|---|
| Theoretical determination of the infrared spectra of amorphous polymers. | The journal of physical chemistry. A 20120712 |
| 5-(2-Chloro-benz-yl)-4,5,6,7-tetra-hydro-thieno[3,2-c]pyridin-2-yl acetate. | Acta crystallographica. Section E, Structure reports online 20120401 |
| Acylation of Chiral Alcohols: A Simple Procedure for Chiral GC Analysis. | Journal of analytical methods in chemistry 20120101 |
| Direct synthesis of anti-1,3-diols through nonclassical reaction of aryl Grignard reagents with isopropenyl acetate. | Organic letters 20110121 |
| Mechanism of acetaldehyde-induced deactivation of microbial lipases. | BMC biochemistry 20110101 |
| Large nonstatistical branching ratio in the dissociation of pentane-2,4-dione radical cation: an ab initio direct classical trajectory study. | The journal of physical chemistry. A 20090226 |
| Inhibitory effect of some acetyl esters and acetamides on glycation of the histone H1. | Zeitschrift fur Naturforschung. C, Journal of biosciences 20080101 |
| Enol esters: versatile substrates for Mannich-type multicomponent reactions. | Organic letters 20071011 |
| Air-stable racemization catalysts for the dynamic kinetic resolution of secondary alcohols. | The Journal of organic chemistry 20070831 |
| Dynamic kinetic resolution of secondary alcohols combining enzyme-catalyzed transesterification and zeolite-catalyzed racemization. | Chemistry (Weinheim an der Bergstrasse, Germany) 20070101 |
| The allosteric modulation of lipases and its possible biological relevance. | Theoretical biology & medical modelling 20070101 |
| Large-scale ruthenium- and enzyme-catalyzed dynamic kinetic resolution of (rac)-1-phenylethanol. | Beilstein journal of organic chemistry 20070101 |
| One-Pot synthesis of gamma,delta-unsaturated carbonyl compounds from allyl alcohols and vinyl or isopropenyl acetates catalyzed by [IrCl(cod)]2. | The Journal of organic chemistry 20060804 |
| Application and comparison of immobilized and coated amylose tris-(3,5-dimethylphenylcarbamate) chiral stationary phases for the enantioselective separation of beta-blockers enantiomers by liquid chromatography. | Talanta 20060115 |
| Stereochemistry of a diastereoisomeric amphiphile and the species of the lipase influence enzyme activity in the transesterification catalyzed by a lipase-co-lyophilizate with the amphiphile in organic media. | Biotechnology letters 20031101 |
| Efficient lipase-catalyzed enantioselective desymmetrization of prochiral 2,2-disubstituted 1,3-propanediols and meso 1,2-diols using 1-ethoxyvinyl 2-furoate. | The Journal of organic chemistry 20020125 |
| Lipase-catalyzed irreversible transesterification of 1-(2-furyl)ethanol using isopropenyl acetate. | Chirality 20010201 |
| Acetyl phosphate synthesis by reaction of isopropenyl acetate and phosphoric acid. | The Journal of biological chemistry 19500801 |