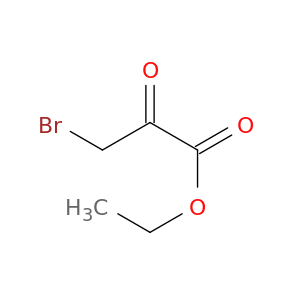
Ethyl 3-bromo-2-oxopropanoate
| Title | Journal |
|---|---|
| Ethyl 8-amino-6-bromoimidazo[1,2-a]pyridine-2-carb-oxy-late. | Acta crystallographica. Section E, Structure reports online 20110601 |
| Ethyl 5-methyl-imidazo[1,2-a]pyridine-2-carboxyl-ate. | Acta crystallographica. Section E, Structure reports online 20100801 |
| Calyculins and related marine natural products as serine-threonine protein phosphatase PP1 and PP2A inhibitors and total syntheses of calyculin A, B, and C. | Marine drugs 20100101 |
| A one-pot synthesis of functionalized thiazoles from acid chlorides, secondary amines, ethyl bromopyruvate, and ammonium thiocyanate. | Molecular diversity 20090801 |
| Variable involvement of the perivascular retinal tissue in carbonic anhydrase inhibitor induced relaxation of porcine retinal arterioles in vitro. | Investigative ophthalmology & visual science 20071001 |
| Synthesis of functionalized 5-imino-2,5-dihydro-furans through the reaction of isocyanides with activated acetylenes in the presence of ethyl bromopyruvate. | Molecular diversity 20060801 |