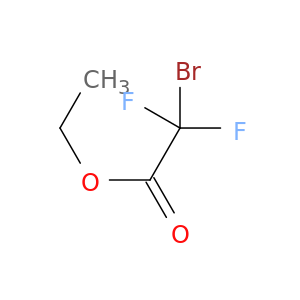
Ethyl Bromodifluoroacetate
| Title | Journal |
|---|---|
| Synthesis of 4-substituted 3,3-difluoropiperidines. | The Journal of organic chemistry 20100205 |
| Addition of difluoromethyl radicals to glycals: a new route to alpha-CF2-D-glycosides. | Organic letters 20070621 |
| Stereocontrolled synthesis of beta-difluoromethylated materials. | The Journal of organic chemistry 20050722 |
| Convenient asymmetric synthesis of beta-substituted alpha,alpha-difluoro-beta-amino acids via Reformatsky reaction between Davis' N-sulfinylimines and ethyl bromodifluoroacetate. | The Journal of organic chemistry 20030919 |
| Asymmetric synthesis of alpha,alpha-difluoro-beta-amino acid derivatives from enantiomerically pure N-tert-butylsulfinimines. | The Journal of organic chemistry 20021115 |