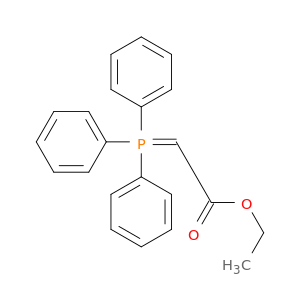
Ethyl (triphenylphosphoranylidene) acetate
| Title | Journal |
|---|---|
| The synthesis of a new class of chiral pincer ligands and their applications in enantioselective catalytic fluorinations and the Nozaki-Hiyama-Kishi reaction. | Chemistry (Weinheim an der Bergstrasse, Germany) 20111223 |
| Enantioselective organocatalytic Michael-Wittig-Michael-Michael reaction: dichotomous construction of pentasubstituted cyclopentanecarbaldehydes and pentasubstituted cyclohexanecarbaldehydes. | Organic letters 20110318 |
| A facile one-pot synthesis of alpha-bromo-alpha,beta-unsaturated esters from alcohols. | Molecules (Basel, Switzerland) 20100504 |
| Ethyl 3-[2-(p-toluene-sulfonamido)phen-yl]acrylate. | Acta crystallographica. Section E, Structure reports online 20091001 |
| Potent new small-molecule inhibitor of botulinum neurotoxin serotype A endopeptidase developed by synthesis-based computer-aided molecular design. | PloS one 20090101 |
| An efficient synthesis of tetramic acid derivatives with extended conjugation from L-ascorbic acid. | Beilstein journal of organic chemistry 20060101 |