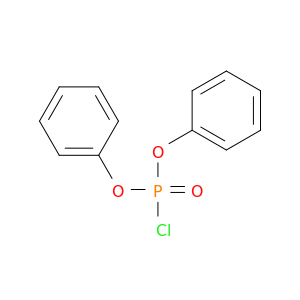
Diphenyl chlorophosphate
| Title | Journal |
|---|---|
| Reactive intermediates in the H-phosphonate synthesis of oligonucleotides. | Organic & biomolecular chemistry 20120814 |
| Aryl H-phosphonates 17: (N-aryl)phosphoramidates of pyrimidine nucleoside analogues and their synthesis, selected properties, and anti-HIV activity. | Journal of medicinal chemistry 20111013 |
| Carbon dioxide as a carbonylating agent in the synthesis of 2-oxazolidinones, 2-oxazinones, and cyclic ureas: scope and limitations. | The Journal of organic chemistry 20100507 |
| Diphenyl (benzyl-amido)phosphate. | Acta crystallographica. Section E, Structure reports online 20100101 |
| Efficient chemical synthesis of both anomers of ADP L-glycero- and D-glycero-D-manno-heptopyranose. | Carbohydrate research 20031114 |
| Synthesis of P(1)-Citronellyl-P(2)-alpha-D-pyranosyl pyrophosphates as potential substrates for the E. coli undecaprenyl-pyrophosphoryl-N-acetylglucoseaminyl transferase MurG. | Bioorganic & medicinal chemistry letters 20011217 |