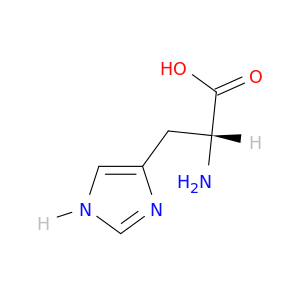
D-Histidine
| Title | Journal |
|---|---|
| Bacillus anthracis spore interactions with mammalian cells: relationship between germination state and the outcome of in vitro. | BMC microbiology 20110101 |
| Carbonic anhydrase activators. The first activation study of a coral secretory isoform with amino acids and amines. | Bioorganic & medicinal chemistry 20100315 |
| Carbonic anhydrase activators: Activation of the beta-carbonic anhydrase from the pathogenic yeast Candida glabrata with amines and amino acids. | Bioorganic & medicinal chemistry letters 20100301 |
| Carbonic anhydrase activators: activation of the beta-carbonic anhydrases from the pathogenic fungi Candida albicans and Cryptococcus neoformans with amines and amino acids. | Bioorganic & medicinal chemistry 20100201 |
| Carbonic anhydrase activators. Activation of the membrane-associated isoform XV with amino acids and amines. | Bioorganic & medicinal chemistry letters 20090701 |
| Carbonic anhydrase activators: activation of the beta-carbonic anhydrase Nce103 from the yeast Saccharomyces cerevisiae with amines and amino acids. | Bioorganic & medicinal chemistry letters 20090315 |
| Carbonic anhydrase activators: activation of the archaeal beta-class (Cab) and gamma-class (Cam) carbonic anhydrases with amino acids and amines. | Bioorganic & medicinal chemistry letters 20081201 |
| Carbonic anhydrase activators: Activation of the human cytosolic isozyme III and membrane-associated isoform IV with amino acids and amines. | Bioorganic & medicinal chemistry letters 20080801 |
| Carbonic anhydrase activators: activation of the human tumor-associated isozymes IX and XII with amino acids and amines. | Bioorganic & medicinal chemistry 20080401 |
| Carbonic anhydrase activators: the first activation study of the human secretory isoform VI with amino acids and amines. | Bioorganic & medicinal chemistry 20070801 |
| Carbonic anhydrase activators: activation of the human isoforms VII (cytosolic) and XIV (transmembrane) with amino acids and amines. | Bioorganic & medicinal chemistry letters 20070801 |
| Carbonic anhydrase activators: an activation study of the human mitochondrial isoforms VA and VB with amino acids and amines. | Bioorganic & medicinal chemistry letters 20070301 |
| Carbonic anhydrase activators. Activation of isozymes I, II, IV, VA, VII, and XIV with l- and d-histidine and crystallographic analysis of their adducts with isoform II: engineering proton-transfer processes within the active site of an enzyme. | Chemistry (Weinheim an der Bergstrasse, Germany) 20060918 |
| The mitochondrial ornithine transporter. Bacterial expression, reconstitution, functional characterization, and tissue distribution of two human isoforms. | The Journal of biological chemistry 20030829 |
| Novel spermine-amino acid conjugates and basic tripeptides enhance cleavage of the hairpin ribozyme at low magnesium ion concentration. | Bioorganic & medicinal chemistry letters 20011203 |
| Effects of intravenous infusion of amino acids and glucose on the yield and concentration of milk protein in dairy cows. | The Journal of dairy research 20010201 |