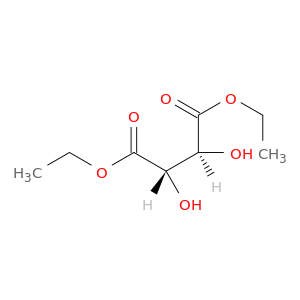
(-)-Diethyl D-tartrate
| Title | Journal |
|---|---|
| Bioplastics from feather quill. | Biomacromolecules 20111010 |
| Biosynthetic chlorination of the piperazate residue in kutzneride biosynthesis by KthP. | Biochemistry 20110712 |
| Total synthesis of broussonetine F: the orthoamide Overman rearrangement of an allylic diol. | Organic letters 20110218 |
| A practical and azide-free synthetic approach to oseltamivir from diethyl D-tartrate. | The Journal of organic chemistry 20100507 |
| Effect of microemulsion component purity on the chromatographic figures of merit in chiral microemulsion electrokinetic chromatography. | Journal of chromatography. A 20090417 |
| Direct chlorination of alcohols with chlorodimethylsilane catalyzed by a gallium trichloride/tartrate system under neutral conditions. | Organic & biomolecular chemistry 20080807 |
| Use of large-scale chromatography in the preparation of armodafinil. | Chirality 20080801 |
| Natural products as an inspiration in the diversity-oriented synthesis of bioactive compound libraries. | Natural product reports 20080801 |
| Synthesis and characterization of mesoporous silica modified with chiral auxiliaries for their potential application as chiral stationary phase. | Journal of chromatography. A 20080516 |
| Separation of corticosteroids by microemulsion EKC with diethyl L-tartrate as the oil phase. | Electrophoresis 20071001 |
| Two-chiral component microemulsion EKC - chiral surfactant and chiral oil. Part 2: diethyl tartrate. | Electrophoresis 20070801 |
| Influence of microemulsion chirality on chromatographic figures of merit in EKC: results with novel three-chiral-component microemulsions and comparison with one- and two-chiral-component microemulsions. | Electrophoresis 20070801 |
| Spectroscopic investigation of the structures of dialkyl tartrates and their cyclodextrin complexes. | The journal of physical chemistry. A 20070208 |
| Synthesis and characterization of (S)-amino alcohol modified M41S as effective material for the enantioseparation of racemic compounds. | Journal of chromatography. A 20061201 |
| Total synthesis of the light-harvesting carotenoid peridinin. | Angewandte Chemie (International ed. in English) 20060612 |
| Total syntheses of naturally occurring diacetylenic spiroacetal enol ethers. | The Journal of organic chemistry 20050722 |
| Bifunctional nanocrystalline MgO for chiral epoxy ketones via Claisen-Schmidt condensation-asymmetric epoxidation reactions. | Journal of the American Chemical Society 20040324 |
| Synthesis and biological characterization of 1alpha,24,25-trihydroxy-2beta-(3-hydroxypropoxy)vitamin D(3) (24-hydroxylated ED-71). | Steroids 20010101 |