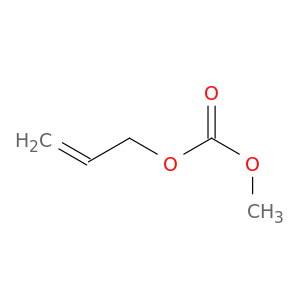
Allyl methyl carbonate
| Title | Journal |
|---|---|
| Palladium(0)-catalyzed stereoselective cyclization of allenenes: divergent synthesis of pyrrolidines and 3-azabicyclo[3.1.0]hexanes from single allenenes. | The Journal of organic chemistry 20040625 |
| Tetrazole synthesis via the palladium-catalyzed three component coupling reaction. | Molecular diversity 20030101 |
| Palladium-catalyzed selective synthesis of 2-allyltetrazoles. | The Journal of organic chemistry 20021018 |
| Synthesis of allyl cyanamides and N-cyanoindoles via the palladium-catalyzed three-component coupling reaction. | Journal of the American Chemical Society 20021009 |